The Medical Device industry plays a pivotal role in modern healthcare, driving innovation in medical technology and enhancing patient outcomes. This sector encompasses a wide range of products, from simple instruments to complex, high-tech devices that monitor and treat various health conditions.
The global market for medical devices is substantial, with significant growth projected due to an aging population, increased chronic diseases, and advancements in technology. According to Deloitte, the medical device market is expected to reach $612 billion by 2025, reflecting the critical role these devices play in the healthcare ecosystem.
Maintaining a robust value chain in the Medical Device sector is crucial for ensuring the delivery of high-quality products, operational efficiency, and compliance with stringent regulatory standards. The Medical Device Value Chain integrates activities from research and development (R&D) and engineering to marketing and regulatory compliance, ensuring seamless operations and timely market delivery.
Effective value chain management enhances operational efficiency, reduces costs, and fosters innovation, thereby creating significant value for patients, healthcare providers, and stakeholders.
Unpacking the Medical Device Value Chain
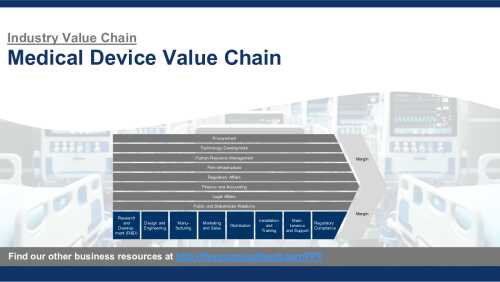
The Medical Device Value Chain encompasses all activities involved in the development, production, and distribution of medical devices. A thorough value chain analysis helps identify opportunities for value creation and operational improvement.

Primary Activities:
- Research and Development (R&D): Innovating new medical devices and improving existing products.
- Design and Engineering: Creating detailed designs and engineering solutions for manufacturing.
- Manufacturing: Producing medical devices with high precision and quality control.
- Marketing and Sales: Promoting products and driving sales through strategic marketing efforts.
- Distribution: Ensuring efficient and timely delivery of medical devices to the market.
- Installation and Training: Installing devices and training healthcare professionals on their use.
- Maintenance and Support: Providing ongoing maintenance and support to ensure device functionality.
- Regulatory Compliance: Ensuring devices meet all regulatory standards and requirements.
Support Activities:
- Procurement: Sourcing materials and components for device production.
- Technology Development: Investing in new technologies to enhance product innovation and manufacturing processes.
- Human Resource Management: Recruiting and developing skilled personnel.
- Firm Infrastructure: Providing the necessary infrastructure to support operations.
- Regulatory Affairs: Navigating the complex regulatory landscape to ensure compliance.
- Finance and Accounting: Managing financial resources and investments.
- Legal Affairs: Handling legal issues, including intellectual property and contracts.
- Public and Stakeholder Relations: Managing relationships with stakeholders and the public.
Understanding these components is crucial for executives in the Medical Device industry. Effective R&D drives innovation, while precise design and engineering ensure product quality. Manufacturing transforms designs into tangible products, and marketing and sales efforts create market demand. Distribution ensures products reach healthcare providers, and installation and training facilitate proper use. Maintenance and support keep devices operational, and regulatory compliance ensures safety and market access.
Support activities underpin the primary activities, providing the necessary infrastructure and resources. Procurement secures essential materials, technology development drives innovation, and human resource management ensures a skilled workforce. Firm infrastructure supports overall operations, regulatory affairs ensure compliance, finance and accounting manage resources, legal affairs handle legal issues, and public and stakeholder relations maintain the organization’s reputation.
By conducting a comprehensive value chain analysis, organizations can identify strengths and areas for improvement, ensuring the Medical Device Value Chain operates efficiently and effectively, ultimately driving value creation and enhancing customer value.
Download an in-depth presentation breaking down all the Medical Device Value Chain activities here.
Precision Engineering: Customizing the Medical Device Value Chain
Customizing the generic industry value chain to fit a specific organization within the Medical Device sector is essential for maximizing efficiency and achieving strategic goals. Each medical device manufacturer operates under unique conditions dictated by its product offerings, market position, and regulatory environment. Tailoring the value chain ensures alignment with these specific factors, leading to enhanced value creation and improved customer value.
Research and Development (R&D)
Tailoring R&D efforts involves focusing on the specific healthcare challenges and technological advancements relevant to the organization. Leveraging patient and clinician feedback, clinical data, and market research allows organizations to develop innovative solutions that address unmet medical needs.
Best Practices:
- User-Centered Design: Incorporate feedback from healthcare professionals and patients early in the development process to ensure the device meets user needs.
- Collaborative Innovation: Partner with academic institutions, research organizations, and technology firms to stay at the forefront of medical advancements.
- Agile Development: Implement agile methodologies to rapidly iterate and refine prototypes based on real-world testing and feedback.
Design and Engineering
Customizing design and engineering processes ensures that medical devices are not only innovative but also manufacturable at scale and compliant with regulatory standards.
Best Practices:
- Compliance by Design: Integrate regulatory requirements into the design phase to ensure compliance from the outset.
- Advanced Prototyping: Use 3D printing and other rapid prototyping technologies to quickly test and refine designs.
- Sustainable Design: Focus on creating environmentally friendly designs that minimize waste and use sustainable materials.
Manufacturing
Tailoring manufacturing processes involves optimizing production techniques and technologies to ensure high quality, cost-effectiveness, and scalability.
Best Practices:
- Lean Manufacturing: Implement lean manufacturing principles to eliminate waste, reduce costs, and improve efficiency.
- Automation and Robotics: Invest in automation and robotics to enhance precision and reduce labor costs.
- Quality Control: Establish robust quality control processes to ensure consistency and compliance with regulatory standards.
Marketing and Sales
Customizing marketing and sales strategies involves targeting specific market segments and emphasizing the unique value propositions of the medical devices.
Best Practices:
- Targeted Marketing: Use data analytics to identify and target key market segments with personalized marketing campaigns.
- Value-Based Selling: Focus on the value and outcomes that the device delivers to patients and healthcare providers.
- Multichannel Strategies: Leverage a mix of digital marketing, traditional advertising, and direct sales efforts to reach potential customers.
Driving Continuous Innovation
Continuous improvement and innovation are the driving forces behind success in the Medical Device industry. Innovation enhances efficiency, reduces costs, and improves product quality, leading to significant value creation and increased customer value.
Importance of Innovation
Innovation is crucial for staying competitive in the rapidly evolving Medical Device landscape. It enables organizations to differentiate their offerings, meet evolving patient and provider expectations, and adapt to regulatory changes. A culture of continuous improvement fosters agility and resilience, allowing organizations to thrive amid market disruptions.
Recent Innovations in the Industry
- Artificial Intelligence (AI) and Machine Learning: AI and machine learning algorithms are transforming medical devices by enabling advanced diagnostics, predictive analytics, and personalized treatment plans. These technologies enhance device functionality and improve patient outcomes.
- 3D Printing: The use of 3D printing in manufacturing medical devices allows for the creation of custom, patient-specific implants and prosthetics. This technology enhances the precision and fit of medical devices, improving patient satisfaction and outcomes.
- Wearable Technology: Innovations in wearable health devices provide continuous monitoring of vital signs, enabling real-time health management. These devices empower patients to take control of their health and provide healthcare providers with valuable data for informed decision-making.
- Telemedicine Integration: Medical devices that integrate with telemedicine platforms enable remote patient monitoring and consultation, expanding access to care and reducing the need for in-person visits. This is particularly beneficial for patients in remote or underserved areas.
Navigating Regulatory Compliance
Ensuring adherence to industry standards and regulations is non-negotiable in the Medical Device sector. Regulatory compliance encompasses a wide range of requirements, including safety standards, quality management systems, and clinical evaluations. Organizations must navigate this complex regulatory landscape to avoid legal issues and maintain their reputation.
Importance of Compliance
Compliance impacts competitiveness by ensuring products meet stringent safety and efficacy standards, which are critical for gaining market approval and consumer trust. Effective compliance management involves staying updated with regulatory changes, implementing robust monitoring systems, and fostering a culture of accountability. Organizations that proactively manage compliance can avoid costly penalties, enhance their brand reputation, and gain a competitive edge in the market.
Impact on Competitiveness
Adhering to regulatory standards also opens opportunities for global market access. Meeting international regulatory requirements allows medical device companies to expand their reach and serve diverse markets, driving growth and value creation. By prioritizing regulatory compliance, organizations not only protect their operations but also position themselves as reliable and trustworthy players in the Medical Device industry.
Best Practices:
- Regulatory Intelligence: Invest in regulatory intelligence tools and services to stay informed about global regulatory changes and trends.
- Integrated Compliance Systems: Implement integrated compliance systems that streamline regulatory processes and ensure all departments adhere to regulations.
- Training and Development: Regularly train employees on regulatory requirements and best practices to maintain a culture of compliance and accountability.
By customizing the value chain, driving continuous innovation, and ensuring regulatory compliance, medical device companies can enhance their value creation and deliver superior customer value.
FAQs
What are the key components of the Medical Device Value Chain?
The Medical Device Value Chain includes Research and Development (R&D), Design and Engineering, Manufacturing, Marketing and Sales, Distribution, Installation and Training, Maintenance and Support, and Regulatory Compliance.
How can medical device companies ensure compliance with regulatory standards?
Medical device companies can ensure compliance by investing in regulatory intelligence tools, implementing integrated compliance systems, and providing regular training and development for employees on regulatory requirements.
Why is innovation crucial in the Medical Device industry?
Innovation is crucial as it enables companies to differentiate their products, meet evolving patient and provider expectations, adapt to regulatory changes, and maintain competitiveness in a rapidly evolving market.
What are some recent innovations in the Medical Device industry?
Recent innovations include the use of Artificial Intelligence (AI) and Machine Learning for advanced diagnostics and personalized treatment plans, 3D printing for custom implants, wearable health devices for continuous monitoring, and telemedicine integration for remote patient care.
How does customizing the value chain enhance value creation in the Medical Device industry?
Customizing the value chain ensures alignment with specific organizational factors such as product offerings, market position, and regulatory environment, leading to enhanced value creation, improved efficiency, and better customer value.
Precision Matters in Medical Devices
Navigating the intricate landscape of the Medical Device industry requires a meticulous approach to every aspect of the value chain. Each segment, from R&D to regulatory compliance, demands attention to detail and a commitment to excellence. By understanding and optimizing these segments, medical device companies can not only enhance operational efficiency but also drive significant value creation for patients, healthcare providers, and stakeholders.
The journey towards innovation and compliance is a continuous one. As technology evolves and patient needs change, so must the strategies and processes within the value chain. Embracing new technologies, fostering a culture of continuous improvement, and staying ahead of regulatory changes are not just best practices but necessities for sustaining growth and maintaining market leadership in the Medical Device industry.
Ultimately, the goal is to deliver high-quality, reliable medical devices that improve patient outcomes and support the broader healthcare ecosystem. By focusing on precision in both engineering and execution, medical device companies can ensure they are well-positioned to meet the challenges and opportunities of this dynamic industry.