Medical Device Value Chain (PowerPoint PPTX Slide Deck)
PowerPoint (PPTX) 32 Slides
BENEFITS OF THIS POWERPOINT DOCUMENT
- Provides a framework for examining the Medical Device Industry.
- Identifies 15 important insights and considerations for Medical Device Value Chain Analysis.
- Provides a detailed breakdown of the impact of Digital Transformation across the Medical Device Value Chain.
HEALTHCARE PPT DESCRIPTION
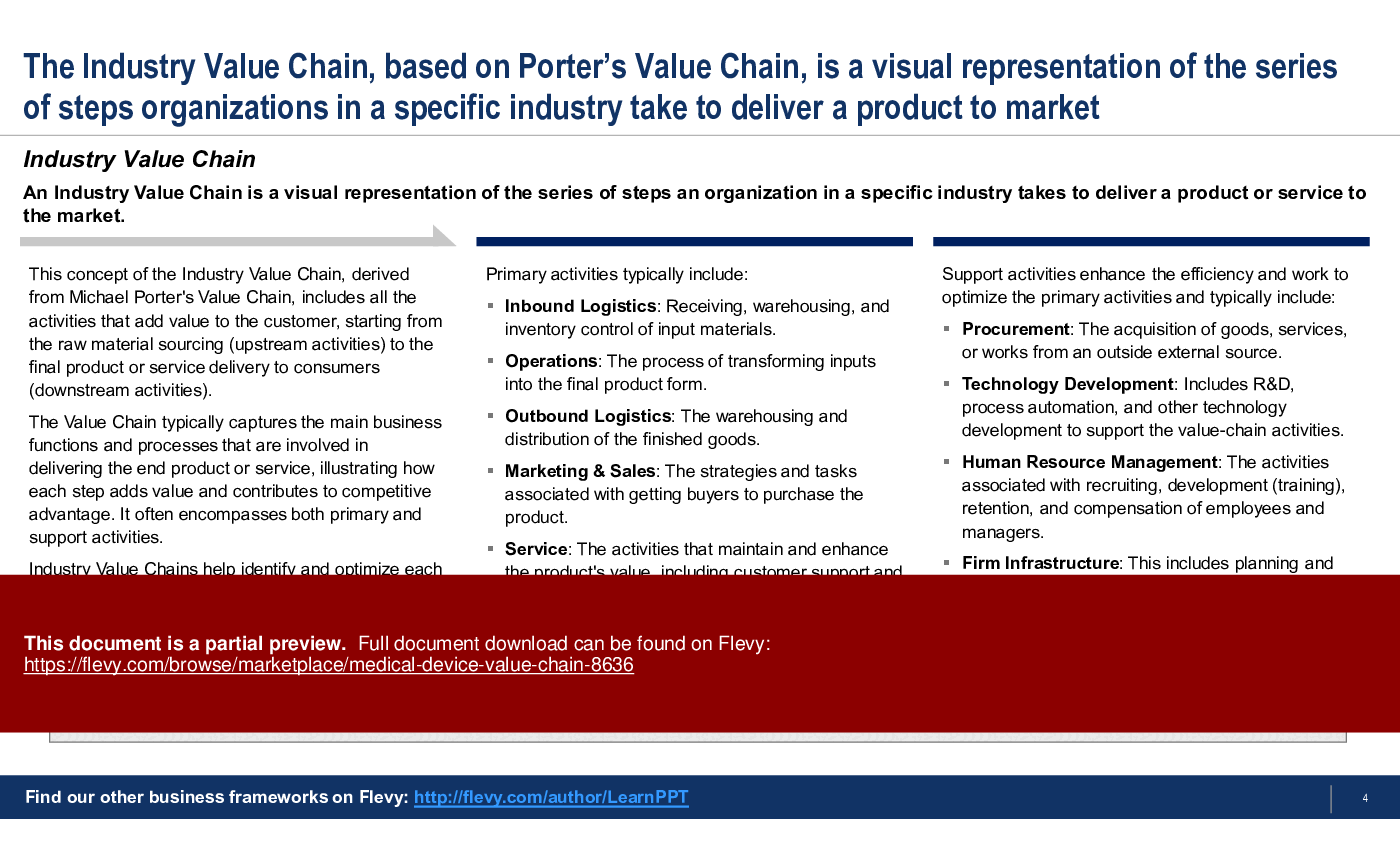
An Industry Value Chain is a visual representation of the series of steps an organization in a specific industry takes to deliver a product or service to the market. It captures the main business functions and processes that are involved in delivering the end product or service, illustrating how each step adds value and contributes to competitive advantage. It often encompasses both primary and support activities.
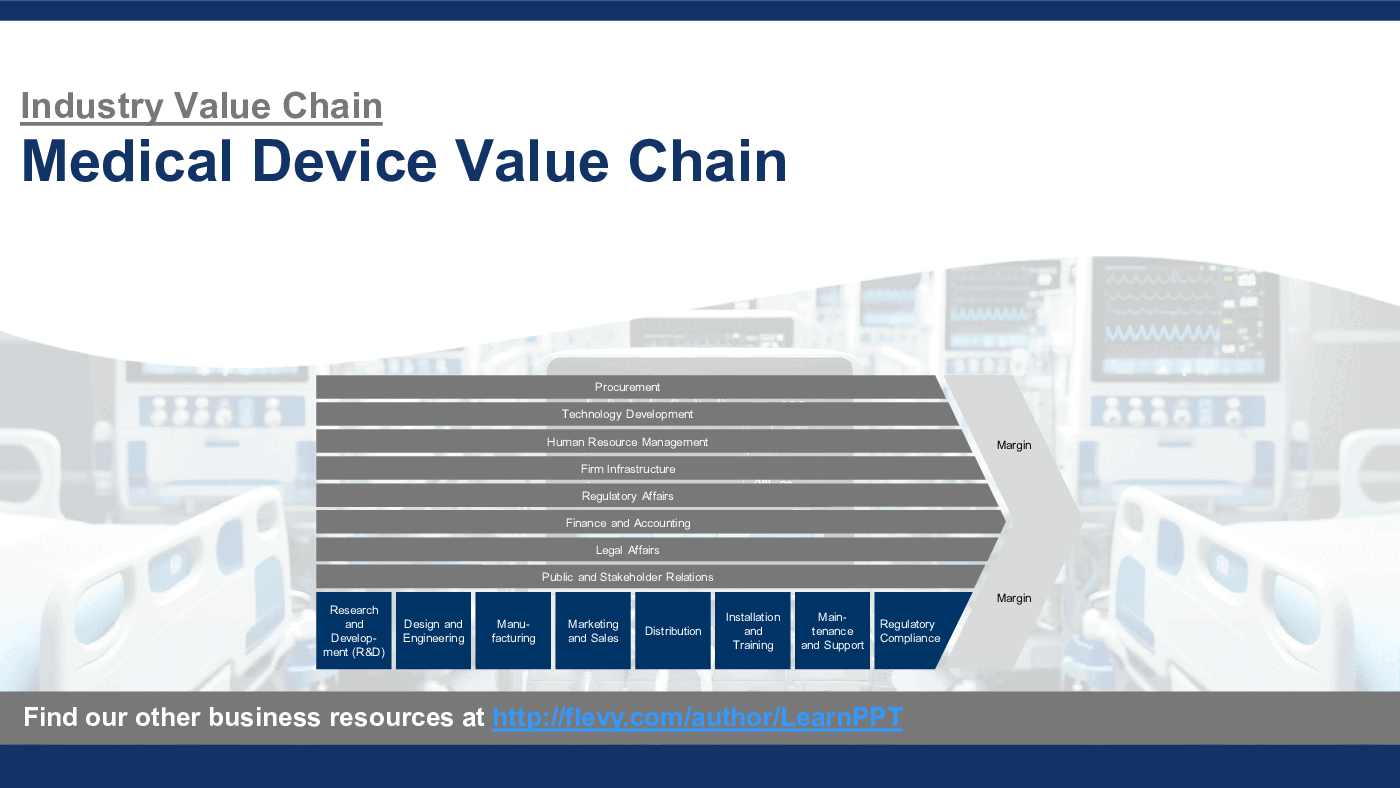
This PowerPoint presentation captures the Medical Device Industry Value Chain, which includes the following primary and support activities for Medical Devices:
Primary Activities
1. Research and Development (R&D)
2. Design and Engineering
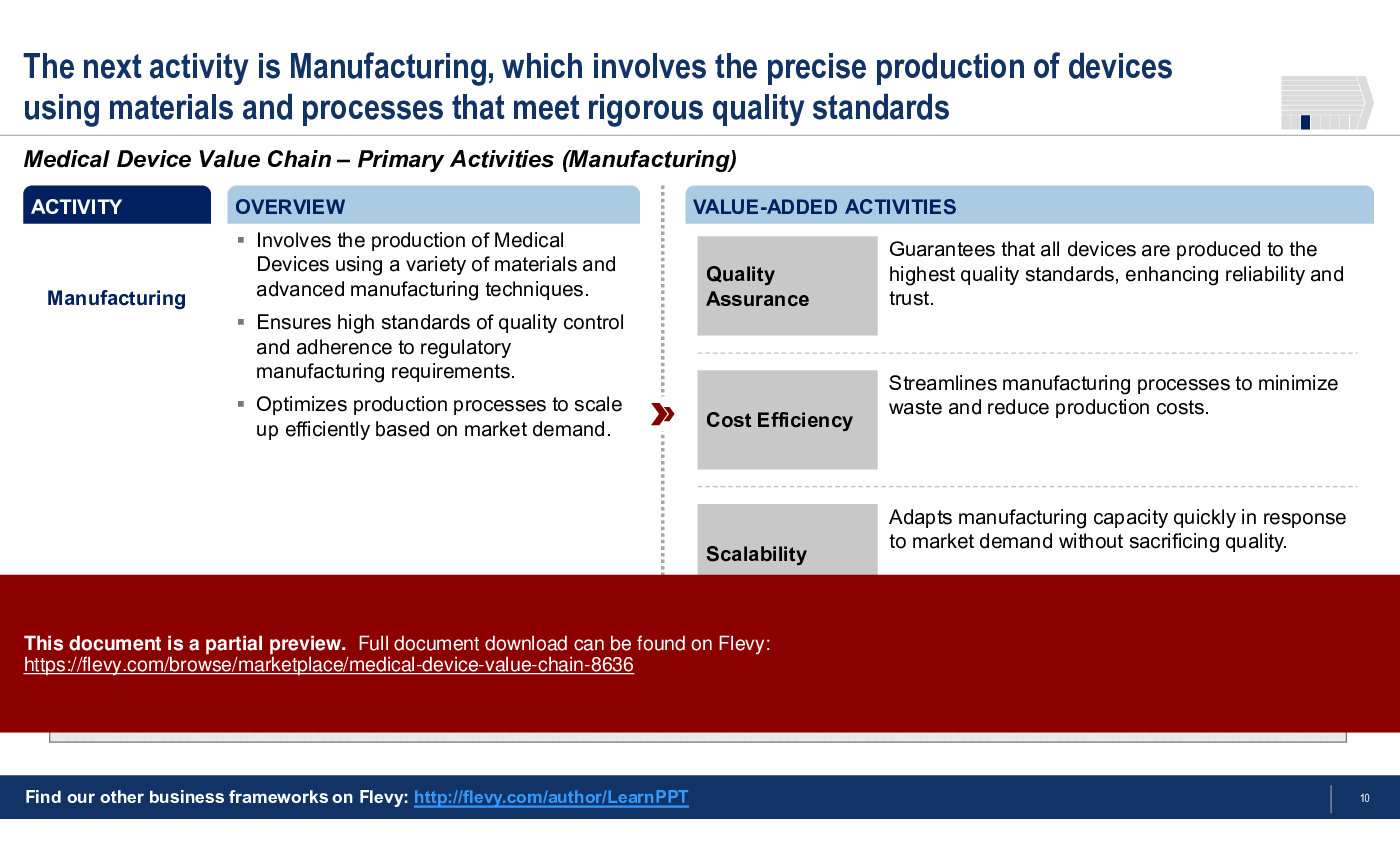
3. Manufacturing
4. Marketing and Sales
5. Distribution
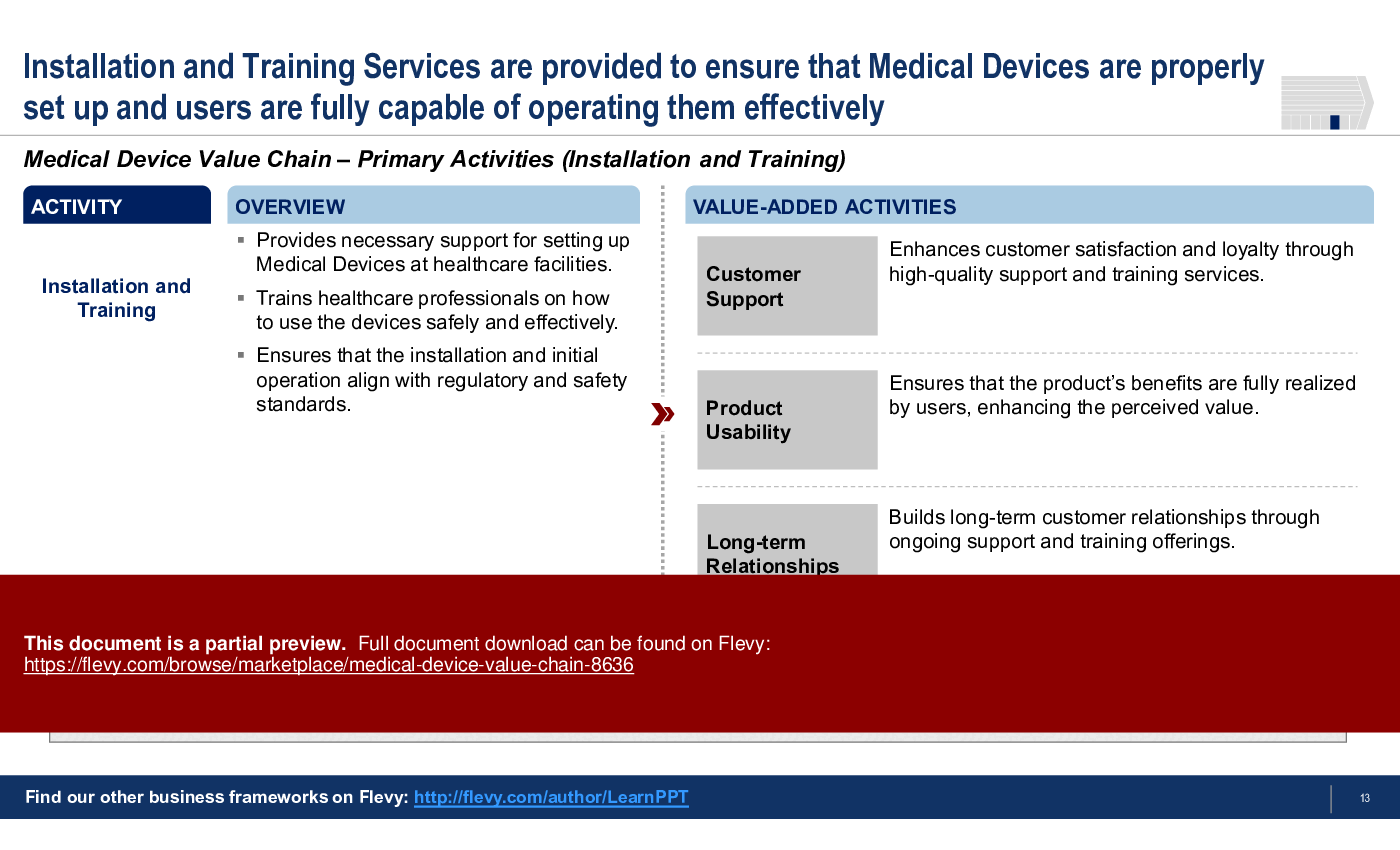
6. Installation and Training
7. Maintenance and Support
8. Regulatory Compliance
Support Activities
1. Procurement
2. Technology Development
3. Human Resource Management
4. Firm Infrastructure
5. Regulatory Affairs
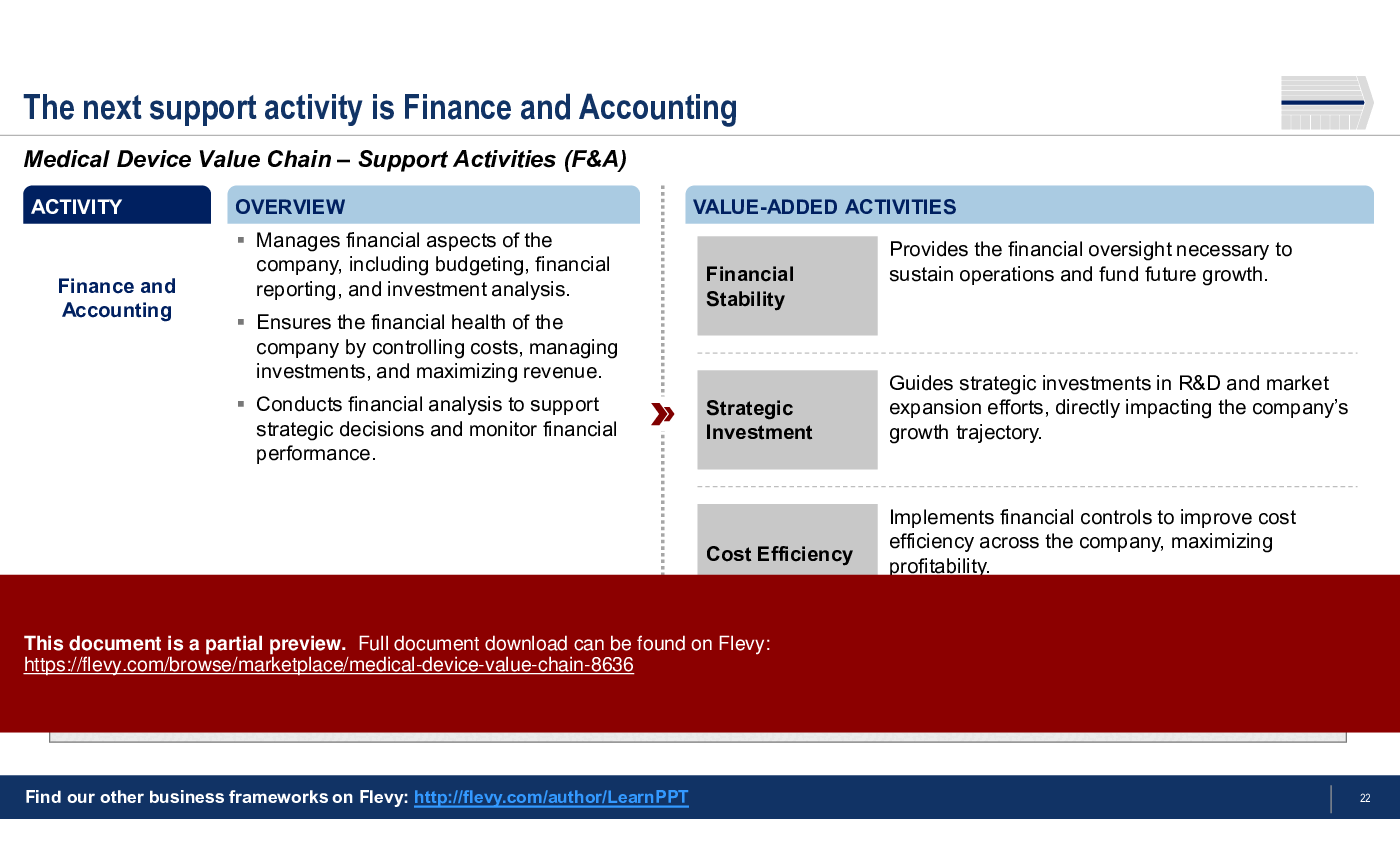
6. Finance and Accounting
7. Legal Affairs
8. Public and Stakeholder Relations
These activities collectively cover the complete lifecycle of Medical Devices from conception to delivery, including the development, production, and market delivery processes, supported by essential back-office functions. The Medical Devices Industry Value Chain integrates complex engineering and rigorous regulatory compliance to deliver innovative products that enhance patient care and operational efficiency in healthcare.
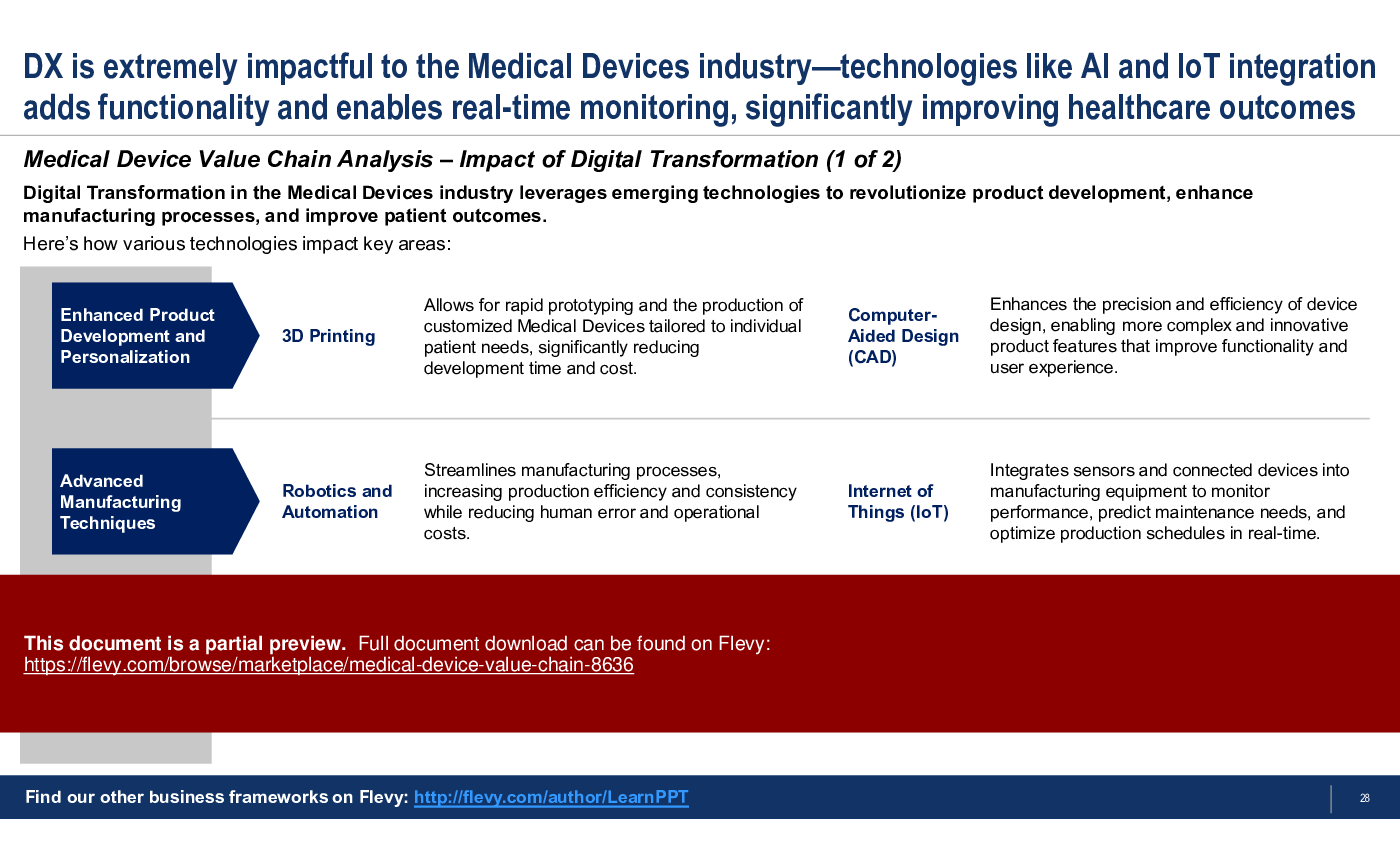
This PowerPoint presentation dives deeper into each of these activities, highlighting key elements. This presentation also discusses Medical Device Value Chain Analysis, highlighting a multitude of key considerations and potential insights to pay attention to. We further discuss the significant impact of Digital Transformation and various specific emergent technologies on the Medical Devices Industry.
Efficient management across the Medical Device Value Chain, from design to distribution, is essential for maintaining the high quality and reliability demanded in Medical Device markets. Strategic collaborations and partnerships within the Value Chain are critical for accelerating product development, streamlining regulatory approvals, and expanding market access.
Got a question about the product? Email us at support@flevy.com or ask the author directly by using the "Ask the Author a Question" form. If you cannot view the preview above this document description, go here to view the large preview instead.
MARCUS OVERVIEW
This synopsis was written by Marcus [?] based on the analysis of the full 32-slide presentation.
Executive Summary
The Medical Device Value Chain presentation provides a detailed framework for understanding the series of steps involved in delivering medical devices to the market. Created by former consultants from top firms such as McKinsey, BCG, and Deloitte, this consulting-grade resource outlines both primary and support activities that contribute to competitive advantage in the medical device industry. Buyers will gain actionable insights into optimizing processes from research and development to distribution and regulatory compliance, enhancing operational efficiency and patient care.
Who This Is For and When to Use
• Executives in the medical device industry seeking to improve operational efficiency
• Product managers focused on R&D and market introduction
• Compliance officers ensuring adherence to regulatory standards
• Marketing and sales teams aiming to enhance market penetration
• Supply chain managers responsible for logistics and distribution
Best-fit moments to use this deck:
• During strategic planning sessions to assess the entire value chain
• For training sessions on optimizing operational processes
• In workshops aimed at improving regulatory compliance strategies
• When evaluating partnerships and collaborations within the value chain
Learning Objectives
• Define the components of the Medical Device Value Chain and their interdependencies
• Analyze the impact of digital transformation on product development and distribution
• Identify key performance indicators for each stage of the value chain
• Develop strategies for enhancing regulatory compliance and market access
• Assess the effectiveness of marketing and sales strategies in the medical device sector
• Implement best practices for procurement and supply chain management
Table of Contents
• Executive Summary (page 1)
• Medical Device Value Chain (page 2)
• Primary Activities (page 3)
• Support Activities (page 4)
• Medical Device Value Chain Analysis (page 5)
Primary Topics Covered
• Research and Development (R&D) - Focuses on innovating and developing new medical devices that meet healthcare needs and regulatory standards.
• Design and Engineering - Translates research findings into manufacturable designs that comply with safety standards.
• Manufacturing - Involves the production of medical devices while ensuring high-quality standards and regulatory compliance.
• Marketing and Sales - Develops strategies to promote medical devices to healthcare providers, emphasizing product benefits.
• Distribution - Manages logistics to ensure timely delivery of medical devices while maintaining quality.
• Installation and Training - Provides support for setting up devices and training users to maximize functionality.
• Maintenance and Support - Offers ongoing technical support to ensure devices operate correctly throughout their lifecycle.
• Regulatory Compliance - Manages compliance with health regulatory agencies to facilitate market access.
Deliverables, Templates, and Tools
• Value chain analysis template for assessing operational efficiency
• R&D effectiveness assessment framework
• Marketing strategy development guide tailored for medical devices
• Regulatory compliance checklist for product approval
• Supply chain optimization model
• Customer engagement and support strategy template
Slide Highlights
• Overview of the Medical Device Value Chain, illustrating primary and support activities
• Detailed breakdown of R&D processes and their impact on innovation
• Insights into effective marketing and sales strategies for medical devices
• Visual representation of regulatory compliance pathways
• Case studies showcasing successful implementation of value chain optimizations
Potential Workshop Agenda
Value Chain Optimization Session (90 minutes)
• Review the Medical Device Value Chain framework
• Identify areas for improvement across primary and support activities
• Develop action plans for enhancing operational efficiency
Regulatory Compliance Deep Dive (60 minutes)
• Discuss current regulatory challenges in the medical device industry
• Explore best practices for maintaining compliance
• Create a roadmap for regulatory submissions
Marketing Strategy Development (90 minutes)
• Analyze current marketing strategies and their effectiveness
• Brainstorm new approaches for market penetration
• Develop a comprehensive marketing plan for upcoming product launches
Customization Guidance
• Tailor the R&D section to reflect specific product lines and market needs
• Adjust the marketing strategies to align with regional market dynamics
• Update regulatory compliance frameworks based on local regulations and standards
• Modify training materials to fit the specific needs of healthcare providers
Secondary Topics Covered
• Digital transformation impacts on the medical device industry
• Cost management strategies within the value chain
• Quality control systems and their importance in manufacturing
• Intellectual property management in the context of medical devices
• Stakeholder engagement strategies for enhancing brand reputation
FAQ
What is the Medical Device Value Chain?
The Medical Device Value Chain is a framework that outlines the series of steps involved in delivering medical devices to the market, encompassing both primary and support activities that add value.
How can this presentation help improve operational efficiency?
The presentation provides insights into optimizing each step of the value chain, from R&D to distribution, enabling organizations to enhance efficiency and reduce costs.
What are the primary activities in the Medical Device Value Chain?
Primary activities include R&D, design and engineering, manufacturing, marketing and sales, distribution, installation and training, maintenance and support, and regulatory compliance.
How does regulatory compliance impact the value chain?
Regulatory compliance ensures that medical devices meet safety and efficacy standards, facilitating market access and reducing the risk of penalties.
What role does digital transformation play in the Medical Device Value Chain?
Digital transformation leverages emerging technologies to enhance product development, improve manufacturing processes, and optimize customer engagement.
What are the key considerations for conducting a value chain analysis?
Key considerations include assessing R&D effectiveness, manufacturing scalability, regulatory compliance strategies, and customer engagement practices.
How can organizations enhance their marketing strategies for medical devices?
Organizations can enhance marketing strategies by utilizing advanced analytics, targeting specific customer segments, and emphasizing product benefits.
What tools are included in this presentation for implementing best practices?
The presentation includes templates and frameworks for value chain analysis, marketing strategy development, and regulatory compliance checklists.
Glossary
• Value Chain - A series of steps an organization takes to deliver a product or service to the market.
• R&D - Research and Development; the process of innovating and developing new products.
• Regulatory Compliance - Adherence to laws and regulations governing product safety and efficacy.
• Supply Chain - The network involved in producing and delivering a product to the end user.
• Market Access - The ability to sell products in a specific market.
• Customer Engagement - The interaction between a company and its customers to enhance satisfaction and loyalty.
• Digital Transformation - The integration of digital technology into all areas of a business.
• Quality Control - Processes to ensure products meet required standards.
• Stakeholder - Any individual or group that has an interest in the success of a company.
• Cost Management - Strategies to control and reduce costs while maintaining quality.
• Intellectual Property - Legal rights to inventions and innovations.
• Marketing Strategy - A plan to promote and sell products effectively.
• Training and Support - Services provided to ensure users can effectively operate products.
• Installation - The process of setting up equipment for use.
• Maintenance - Ongoing support to ensure product functionality.
• Public Relations - Managing the company’s image and communications with the public.
• Firm Infrastructure - The organizational structure and systems that support business operations.
• Human Resource Management - The process of recruiting, training, and managing employees.
• Technology Development - The integration of new technologies to improve products and processes.
• Procurement - The process of acquiring goods and services.
HEALTHCARE PPT SLIDES
Source: Best Practices in Healthcare, Value Chain Analysis PowerPoint Slides: Medical Device Value Chain PowerPoint (PPTX) Presentation Slide Deck, LearnPPT Consulting