Pharmaceuticals Value Chain (PowerPoint PPTX Slide Deck)
PowerPoint (PPTX) 25 Slides
BENEFITS OF THIS POWERPOINT DOCUMENT
- Provides a framework for examining the Pharmaceuticals Industry.
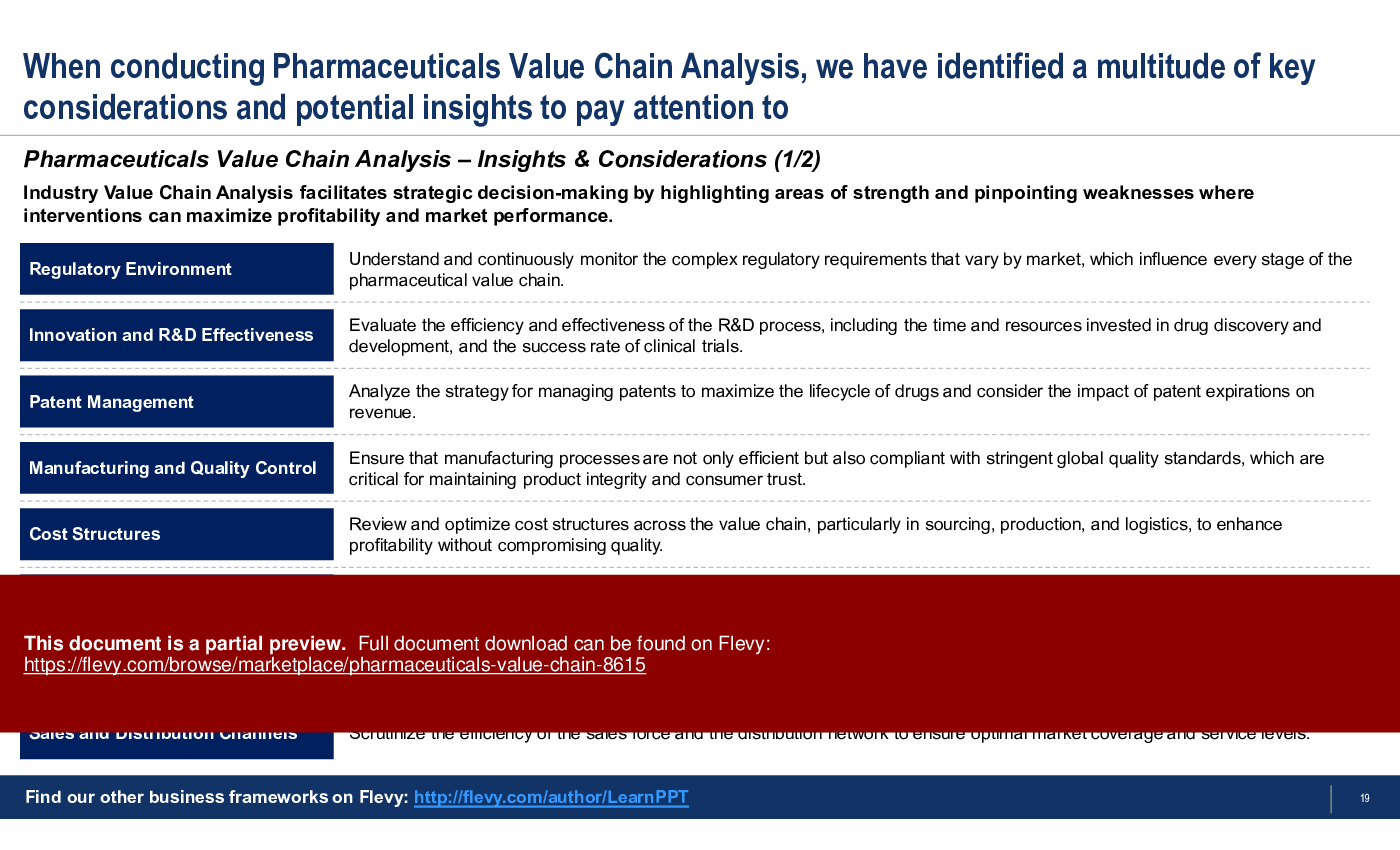
- Identifies 15 important insights and considerations for Pharma Value Chain Analysis.
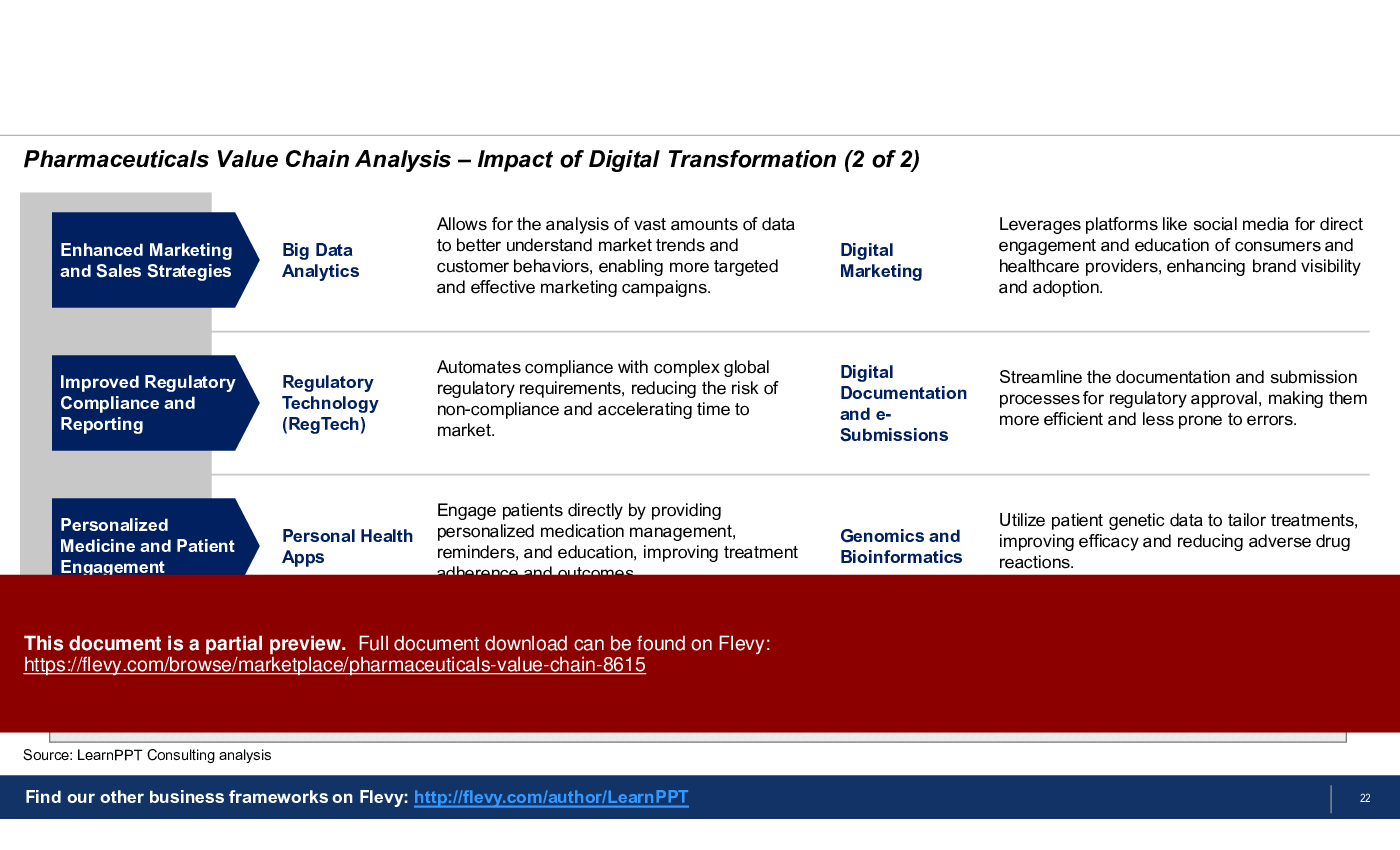
- Provides a detailed breakdown of the impact of Digital Transformation across the Pharma Value Chain.
HEALTHCARE PPT DESCRIPTION
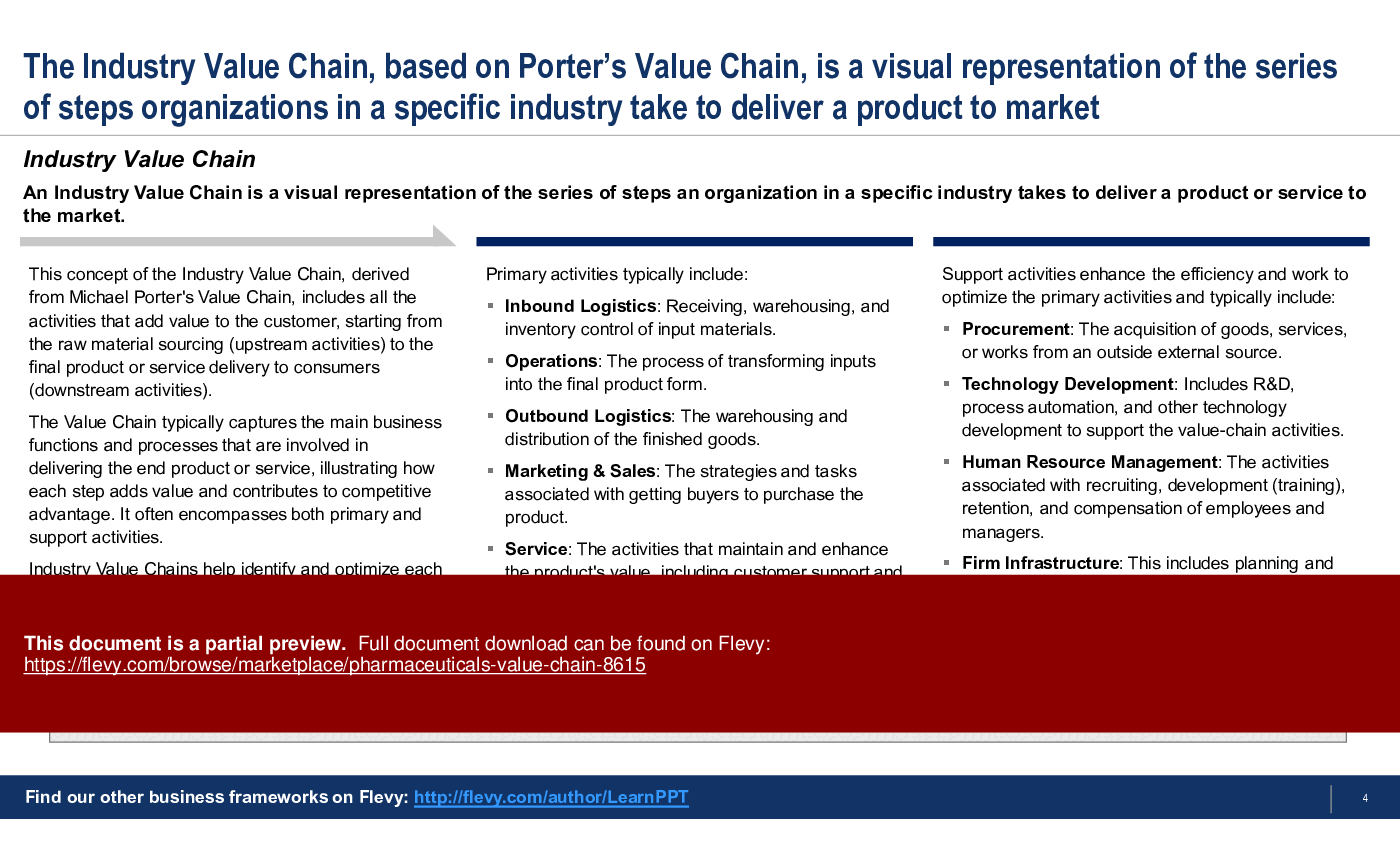
An Industry Value Chain is a visual representation of the series of steps an organization in a specific industry takes to deliver a product or service to the market. It captures the main business functions and processes that are involved in delivering the end product or service, illustrating how each step adds value and contributes to competitive advantage. It often encompasses both primary and support activities.
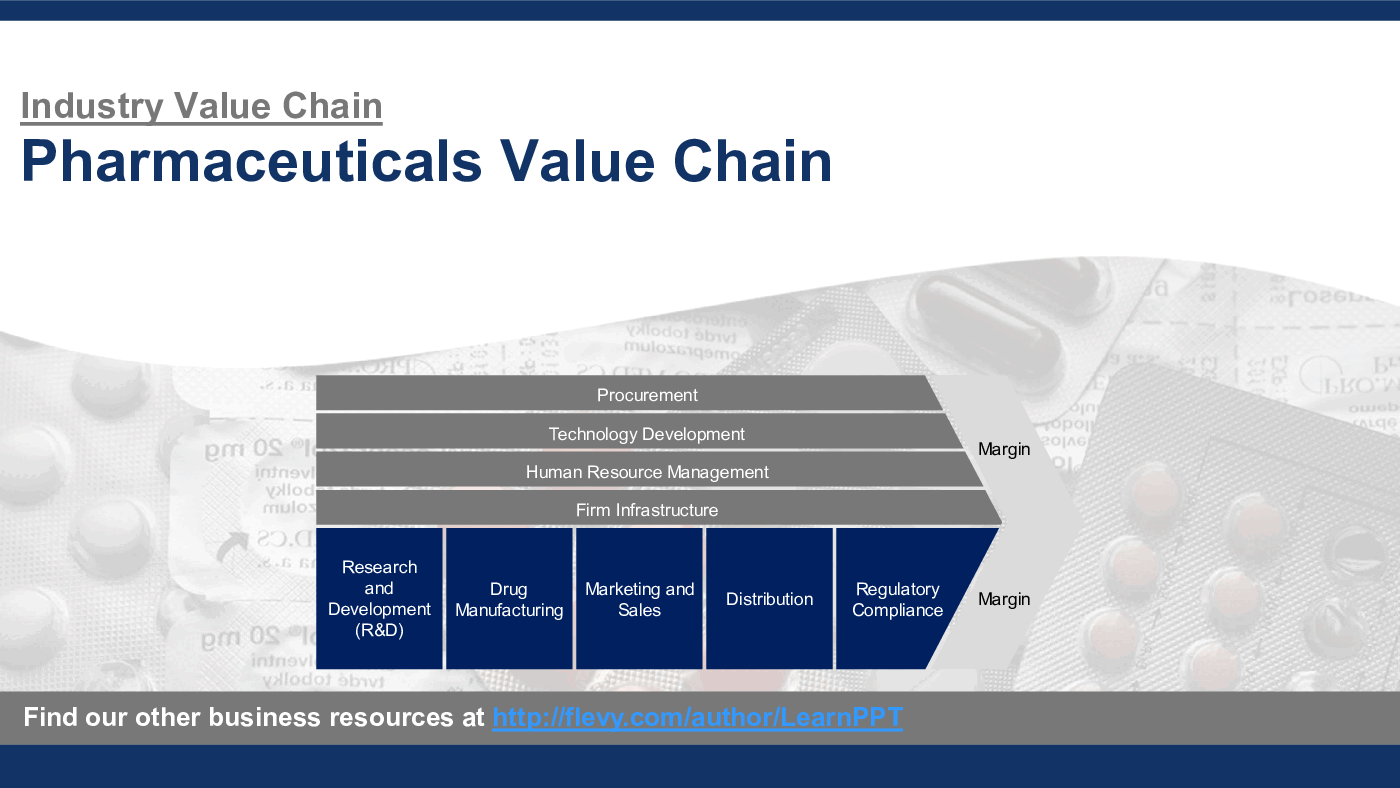
This PowerPoint presentation captures the Pharmaceuticals Industry Value Chain, which includes the following primary and support activities:
Primary Activities
1. Research and Development (R&D)
2. Drug Manufacturing
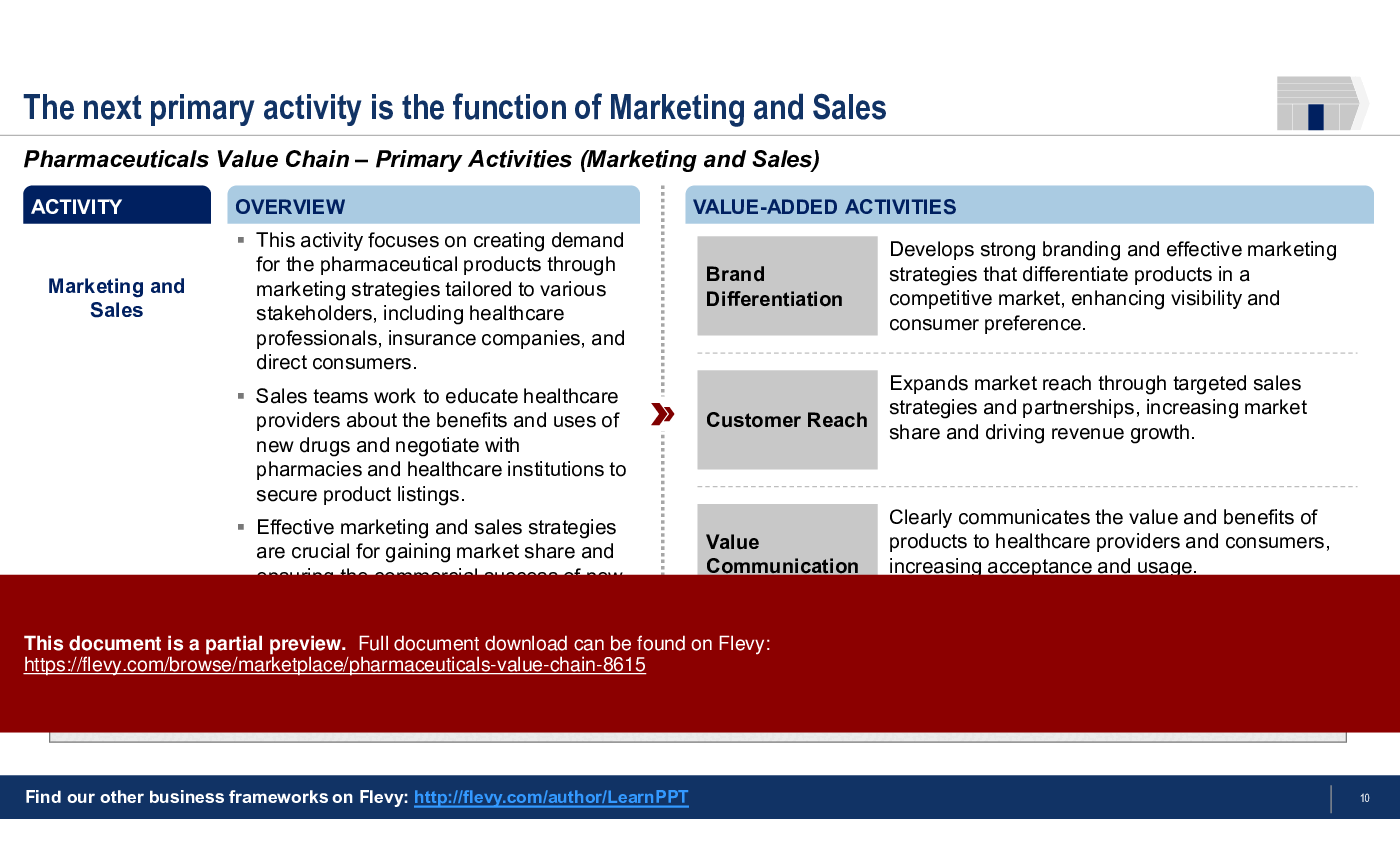
3. Marketing and Sales
4. Distribution
5. Regulatory Compliance
Support Activities
1. Procurement
2. Technology Development
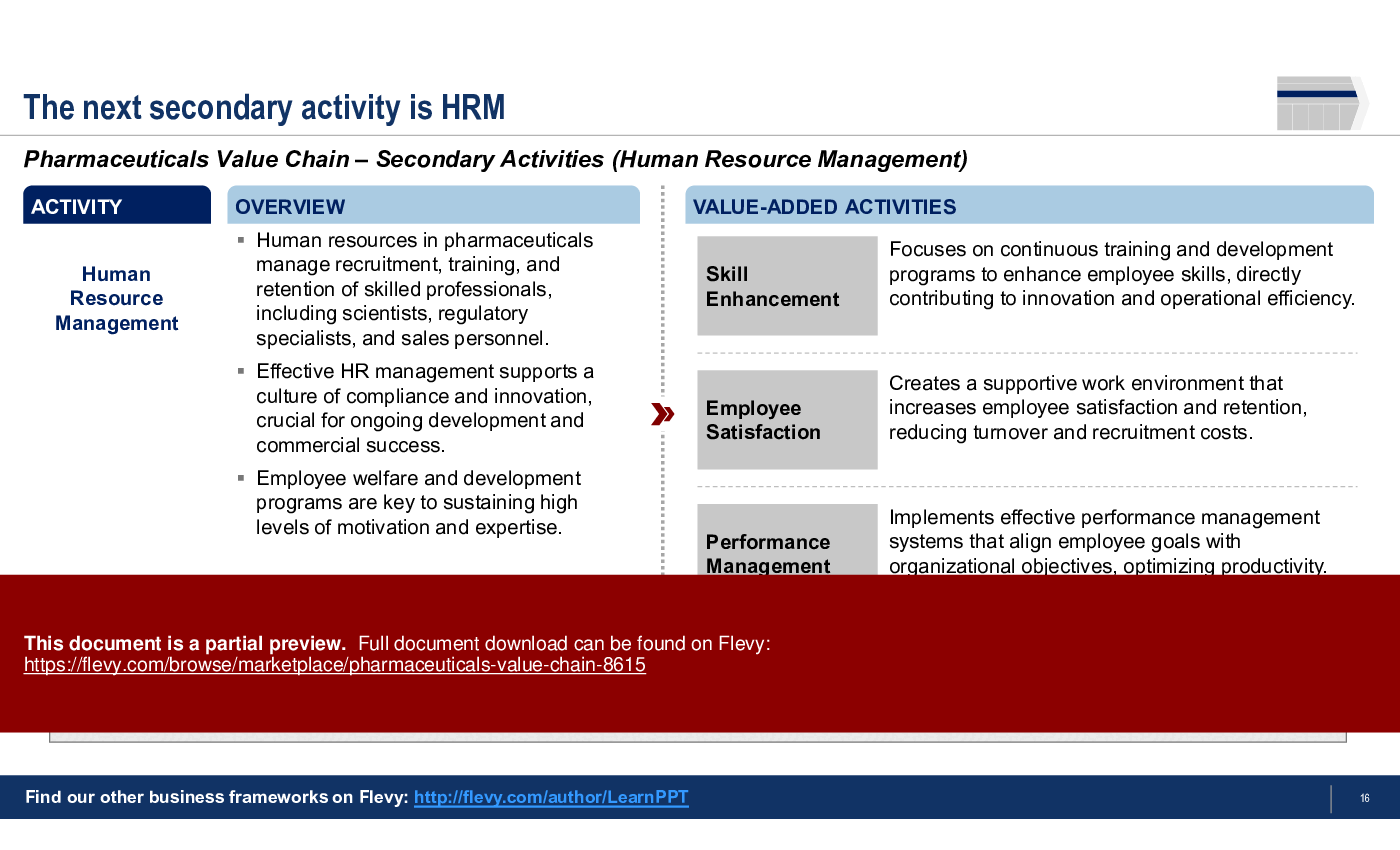
3. Human Resource Management
4. Firm Infrastructure
Each of these activities is critical to the smooth operation and success of a pharmaceutical company, and excellence in each area can lead to Competitive Advantages in a highly competitive market.
This PowerPoint presentation dives deeper into each of these activities, highlighting key elements. This presentation also discusses Pharmaceuticals Value Chain Analysis, highlighting a multitude of key considerations and potential insights to pay attention to. We further discuss the significant impact of Digital Transformation and various specific emergent technologies on the Pharma Industry.
The Pharmaceutical Industry Value Chain integrates complex R&D processes with stringent regulatory environments, requiring a delicate balance of innovation, compliance, and market dynamics to deliver effective treatments. Efficiency in the Pharma Value Chain, particularly in drug manufacturing and distribution, directly impacts global health outcomes by determining how quickly and widely new treatments can reach patients. Strategic collaborations and partnerships within the value chain are essential for enhancing drug discovery and development efficiencies, reducing costs, and speeding up time-to-market.
This presentation provides a comprehensive overview of the Pharmaceuticals Value Chain, detailing essential activities that drive operational success. It emphasizes the importance of aligning marketing strategies with regulatory compliance to enhance market reach and ensure product integrity.
Got a question about the product? Email us at support@flevy.com or ask the author directly by using the "Ask the Author a Question" form. If you cannot view the preview above this document description, go here to view the large preview instead.
MARCUS OVERVIEW
This synopsis was written by Marcus [?] based on the analysis of the full 25-slide presentation.
Executive Summary
The Pharmaceuticals Value Chain presentation offers a detailed exploration of the critical steps involved in delivering pharmaceutical products to market, structured in a manner akin to McKinsey, Bain, or BCG-quality consulting frameworks (consulting-grade; not affiliated). This resource elucidates both primary and support activities within the pharmaceutical industry, providing insights into how each step contributes to competitive advantage. By leveraging this framework, executives and consultants can enhance operational efficiency, optimize resource allocation, and improve overall market responsiveness.
Who This Is For and When to Use
• Pharmaceutical executives overseeing R&D, manufacturing, and distribution operations
• Strategy consultants focused on market access and competitive analysis
• Supply chain managers aiming to streamline logistics and compliance
• Marketing teams tasked with developing effective product launch strategies
Best-fit moments to use this deck:
• During strategic planning sessions to identify strengths and weaknesses in the value chain
• For training workshops aimed at enhancing team understanding of industry processes
• When evaluating potential partnerships or collaborations within the pharmaceutical sector
Learning Objectives
• Define the components of the Pharmaceuticals Value Chain and their interdependencies
• Analyze the impact of regulatory compliance on operational efficiency
• Develop strategies for optimizing R&D processes and drug development timelines
• Assess the effectiveness of marketing and sales strategies in reaching target audiences
• Identify opportunities for cost reduction across the value chain
• Explore the role of technology in enhancing drug discovery and manufacturing processes
Table of Contents
• Executive Summary (page 3)
• Pharmaceuticals Value Chain (page 4)
• Primary Activities (page 5)
• Support Activities (page 6)
• Pharmaceuticals Value Chain Analysis (page 19)
Primary Topics Covered
• Research and Development (R&D) - R&D serves as the cornerstone of pharmaceutical innovation, encompassing the discovery and development of new drug compounds through rigorous testing and collaboration.
• Drug Manufacturing - This activity focuses on the large-scale production of pharmaceuticals, ensuring compliance with quality and regulatory standards throughout the manufacturing process.
• Marketing and Sales - Strategies aimed at creating demand for pharmaceutical products through targeted marketing efforts and effective communication with healthcare providers.
• Distribution - Involves the logistics of delivering pharmaceuticals to various stakeholders while ensuring regulatory compliance and product integrity.
• Regulatory Compliance - Encompasses all activities necessary to meet legal standards, including documentation for drug approval and ongoing safety reporting.
Deliverables, Templates, and Tools
• Value chain analysis template for assessing operational efficiency
• R&D process optimization framework to enhance drug development timelines
• Marketing strategy playbook tailored for pharmaceutical products
• Compliance checklist for regulatory submissions and approvals
• Supply chain management model focused on logistics optimization
Slide Highlights
• Visual representation of the Pharmaceuticals Value Chain illustrating primary and support activities
• Detailed breakdown of R&D processes and their impact on innovation
• Overview of drug manufacturing standards and compliance requirements
• Insights into effective marketing strategies for pharmaceutical products
• Analysis of distribution networks and their role in customer satisfaction
Potential Workshop Agenda
Pharmaceutical Value Chain Overview (60 minutes)
• Introduce the Pharmaceuticals Value Chain framework and its significance
• Discuss the interdependencies between primary and support activities
• Identify key challenges and opportunities within the value chain
R&D and Manufacturing Deep Dive (90 minutes)
• Explore best practices in R&D and drug manufacturing
• Analyze case studies on successful product development
• Facilitate group discussions on optimizing operational efficiency
Marketing and Distribution Strategies (60 minutes)
• Review effective marketing tactics for pharmaceutical products
• Discuss distribution challenges and solutions in the current market
• Engage participants in brainstorming sessions for innovative approaches
Customization Guidance
• Tailor the value chain analysis to specific organizational contexts and market conditions
• Adjust R&D and manufacturing frameworks to reflect internal processes and technologies
• Incorporate company-specific marketing strategies and distribution channels into the presentation
Secondary Topics Covered
• The impact of digital transformation on the pharmaceutical value chain
• Strategies for managing patent lifecycles and maximizing drug revenue
• Best practices for stakeholder engagement and communication
• Sustainability practices within the pharmaceutical industry
FAQ
What is the Pharmaceuticals Value Chain?
The Pharmaceuticals Value Chain is a framework that outlines the series of steps involved in delivering pharmaceutical products to market, including R&D, manufacturing, marketing, distribution, and regulatory compliance.
How does regulatory compliance impact the value chain?
Regulatory compliance is integral to every phase of the value chain, ensuring that products meet legal standards and maintaining market authorization, which is crucial for patient safety and market stability.
What are the primary activities in the Pharmaceuticals Value Chain?
The primary activities include Research and Development (R&D), Drug Manufacturing, Marketing and Sales, Distribution, and Regulatory Compliance. Each plays a critical role in delivering effective pharmaceutical products.
How can technology enhance the Pharmaceuticals Value Chain?
Investments in technology, such as AI and data analytics, can streamline R&D processes, improve manufacturing efficiency, and enhance marketing strategies, ultimately driving innovation and cost reductions.
What are the key considerations for conducting a Pharmaceuticals Value Chain Analysis?
Key considerations include evaluating regulatory environments, assessing R&D effectiveness, optimizing manufacturing processes, and analyzing marketing strategies to enhance profitability and market performance.
How can organizations improve their supply chain robustness?
Organizations can enhance supply chain robustness by developing strategic partnerships, implementing advanced tracking systems, and ensuring compliance with regulatory requirements to mitigate disruptions.
What role does marketing play in the Pharmaceuticals Value Chain?
Marketing is essential for creating demand, educating healthcare providers, and communicating product value, which directly impacts market share and revenue growth.
How can companies balance global efficiencies with local market needs?
Companies should adopt a hybrid strategy that leverages global operational efficiencies while allowing for local adaptations to meet specific regulatory and consumer demands.
Glossary
• Pharmaceutical Value Chain - A framework outlining the steps involved in delivering pharmaceutical products to market.
• R&D - Research and Development; the process of discovering and developing new drugs.
• Regulatory Compliance - Adherence to laws and regulations governing pharmaceutical products.
• Supply Chain - The network involved in the production and distribution of pharmaceuticals.
• Market Access - Strategies for ensuring that products reach consumers effectively.
• Digital Transformation - The integration of digital technology into all areas of business operations.
• Stakeholder Engagement - The process of involving all parties affected by pharmaceutical products in decision-making.
• Patent Management - Strategies for managing drug patents to maximize revenue.
• Quality Control - Processes ensuring that pharmaceutical products meet required standards.
• Cost Structure - The various costs associated with the production and distribution of pharmaceuticals.
• Market Dynamics - The forces that impact the supply and demand of pharmaceutical products.
• Operational Efficiency - The ability to deliver products effectively while minimizing costs.
• Customer Satisfaction - The measure of how products meet or exceed consumer expectations.
• Innovation - The process of developing new ideas or products in the pharmaceutical industry.
• Compliance Framework - The structure that ensures adherence to regulatory standards.
• Data Management - The process of collecting, storing, and utilizing data effectively.
• Marketing Strategies - Plans developed to promote and sell pharmaceutical products.
• Distribution Network - The system used to deliver products to consumers.
• Sustainability Practices - Efforts to minimize environmental impact in pharmaceutical operations.
• Technology Development - The process of creating and implementing new technologies in drug development and production.
• Human Resource Management - The management of personnel involved in pharmaceutical operations.
• Firm Infrastructure - The organizational structure and systems that support business operations.
HEALTHCARE PPT SLIDES
Source: Best Practices in Healthcare, Value Chain Analysis PowerPoint Slides: Pharmaceuticals Value Chain PowerPoint (PPTX) Presentation Slide Deck, LearnPPT Consulting