ISO 13485:2016 (Medical Devices - QMS) Awareness Training (PowerPoint PPTX Slide Deck)
PowerPoint (PPTX) 67 Slides
BENEFITS OF THIS POWERPOINT DOCUMENT
- Provides a framework for managing and improving the quality management system based on the ISO 13485:2016 standard for the medical devices industry.
- Provides a tool for creating awareness of the ISO 13485:2016 standard.
- Provides guidelines and practical tips for handling an audit session.
QUALITY MANAGEMENT PPT DESCRIPTION
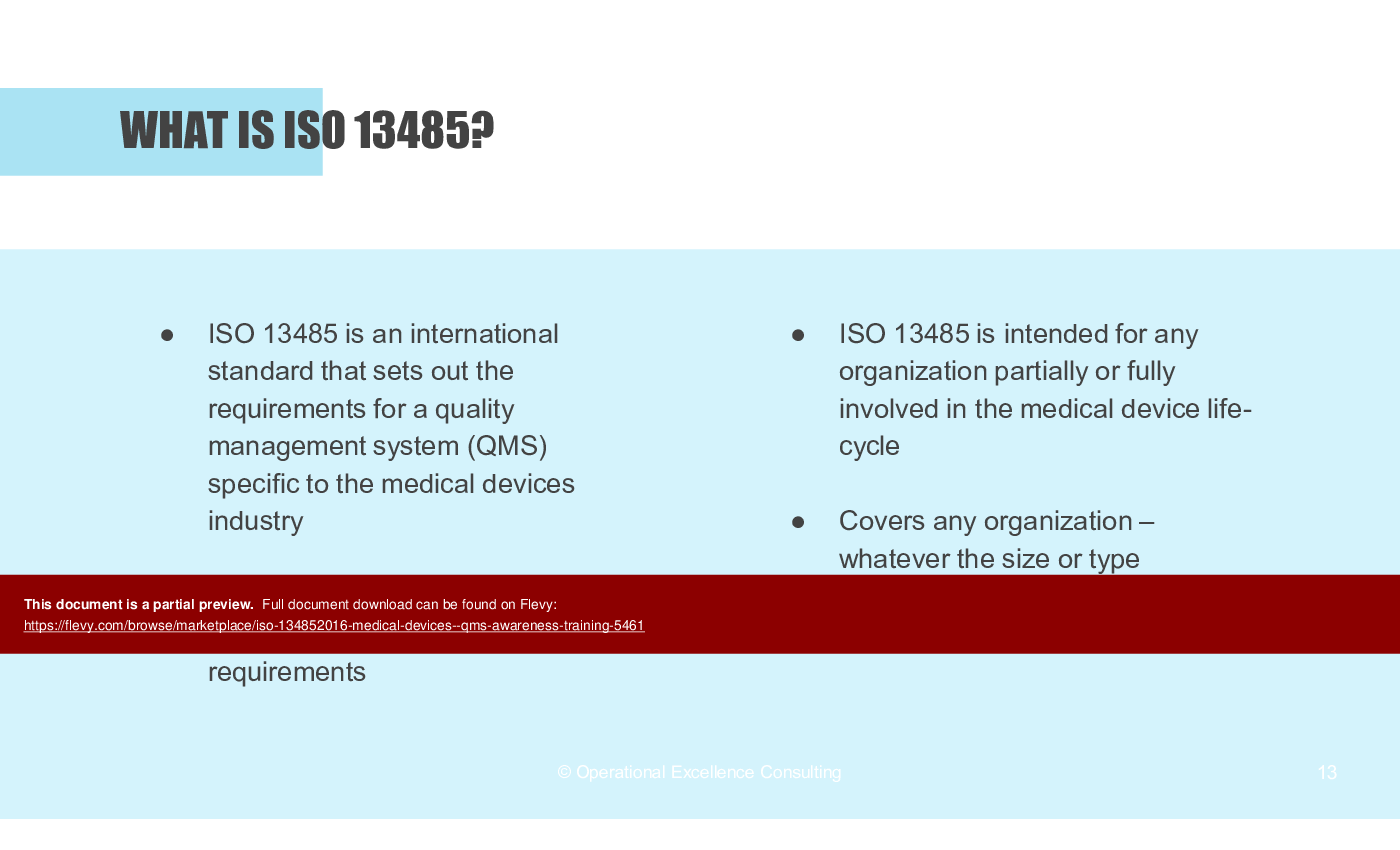
ISO 13485:2016 is an internationally recognized standard that outlines the requirements for a quality management system (QMS) specific to the medical device industry. The standard focuses on meeting customer and regulatory requirements and is intended for any organization involved in the medical device life-cycle.
The standard covers all stages of the medical device life-cycle, from design and development to production, distribution, installation, and servicing. It also includes the provision of associated services and the final decommissioning and disposal of the device.
ISO 13485:2016 helps organizations establish and maintain an effective QMS, ensuring the safety and effectiveness of their medical devices while meeting regulatory requirements. It also reflects a strong commitment to continuous improvement, giving customers confidence in the organization's ability to bring safe and effective products to market.
This ISO 13485 awareness training presentation is designed to provide an introduction to the ISO 13485:2016 standard for employees, new hires, potential auditees, and other stakeholders in the medical device industry. It can also be used as a supplement to the training of quality assurance professionals and internal auditors.
The presentation covers the key clauses of the standard, the audit approach, and offers practical tips on how to handle an audit session. It also emphasizes the importance of meeting customer and regulatory requirements, establishing an effective QMS, and ensuring the safety and effectiveness of medical devices.
By the end of the training, participants will be more informed and comfortable with ISO 13485:2016, enabling them to contribute to the development and maintenance of an effective QMS and the production of safe and effective medical devices.
LEARNING OBJECTIVES
1. Provide background knowledge of ISO 13485:2016.
2. Gain an overview of ISO 13485:2016 structure and the certification process.
3. Understand the audit approach.
4. Gather useful tips for handling an audit session.
CONTENTS
1. Overview of ISO 13485
• About ISO
• ISO Standards Contribute Directly to the U.N. Sustainable Development Goals (SDGs)
• What are Standards?
• What Standards are Not
• Why are Standards Important?
• What is a Management System?
• What is ISO 13485?
• Who is ISO 13485 For?
• What is a Medical Device?
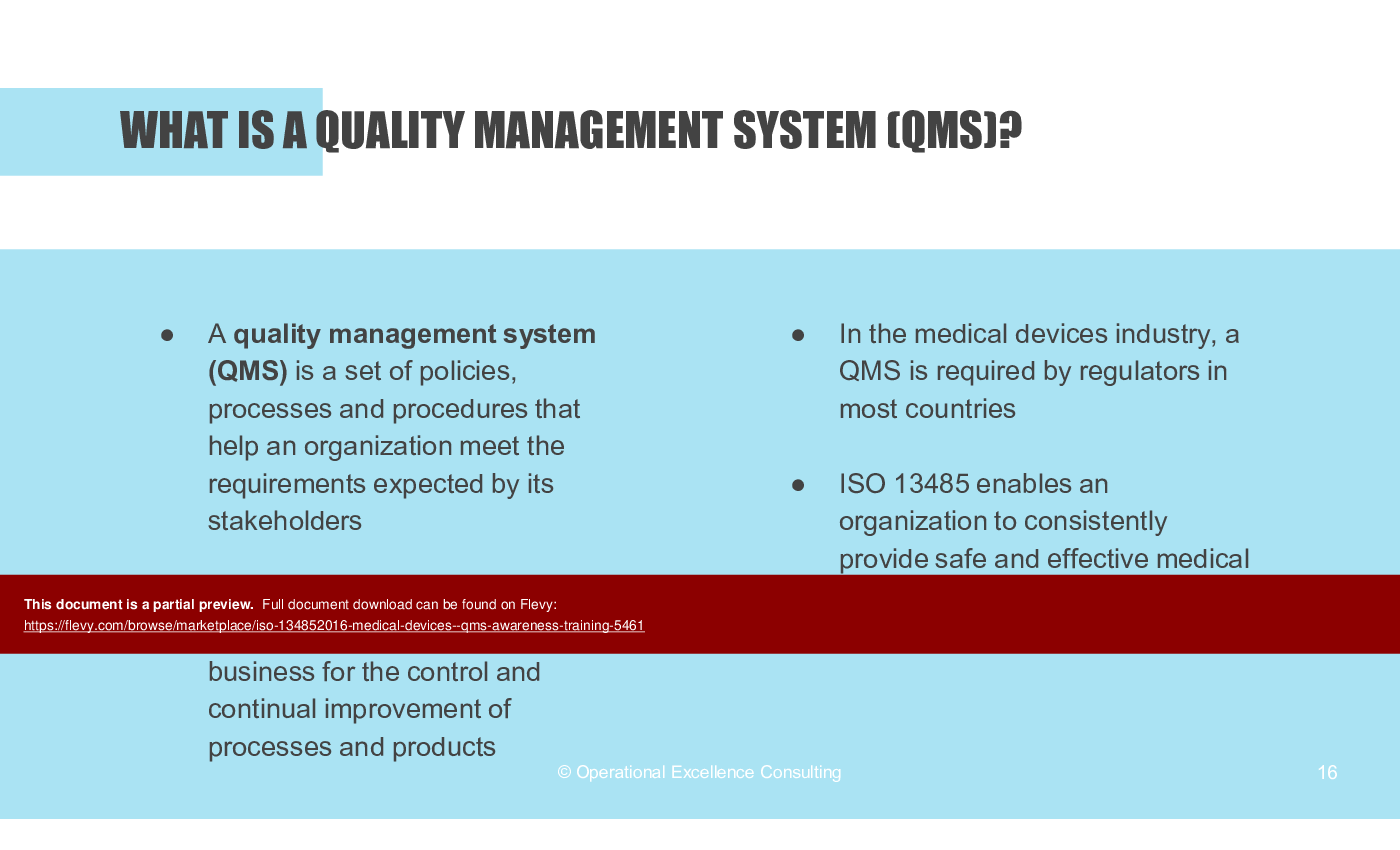
• What is a Quality Management System (QMS)?
• The Plan-Do-Check-Act (PDCA) Process Model
• How Does ISO 13485 Work?
• Benefits that ISO 13485 Will Bring to the Organization
• Advantages of Certification
• Accreditation & Certification Bodies
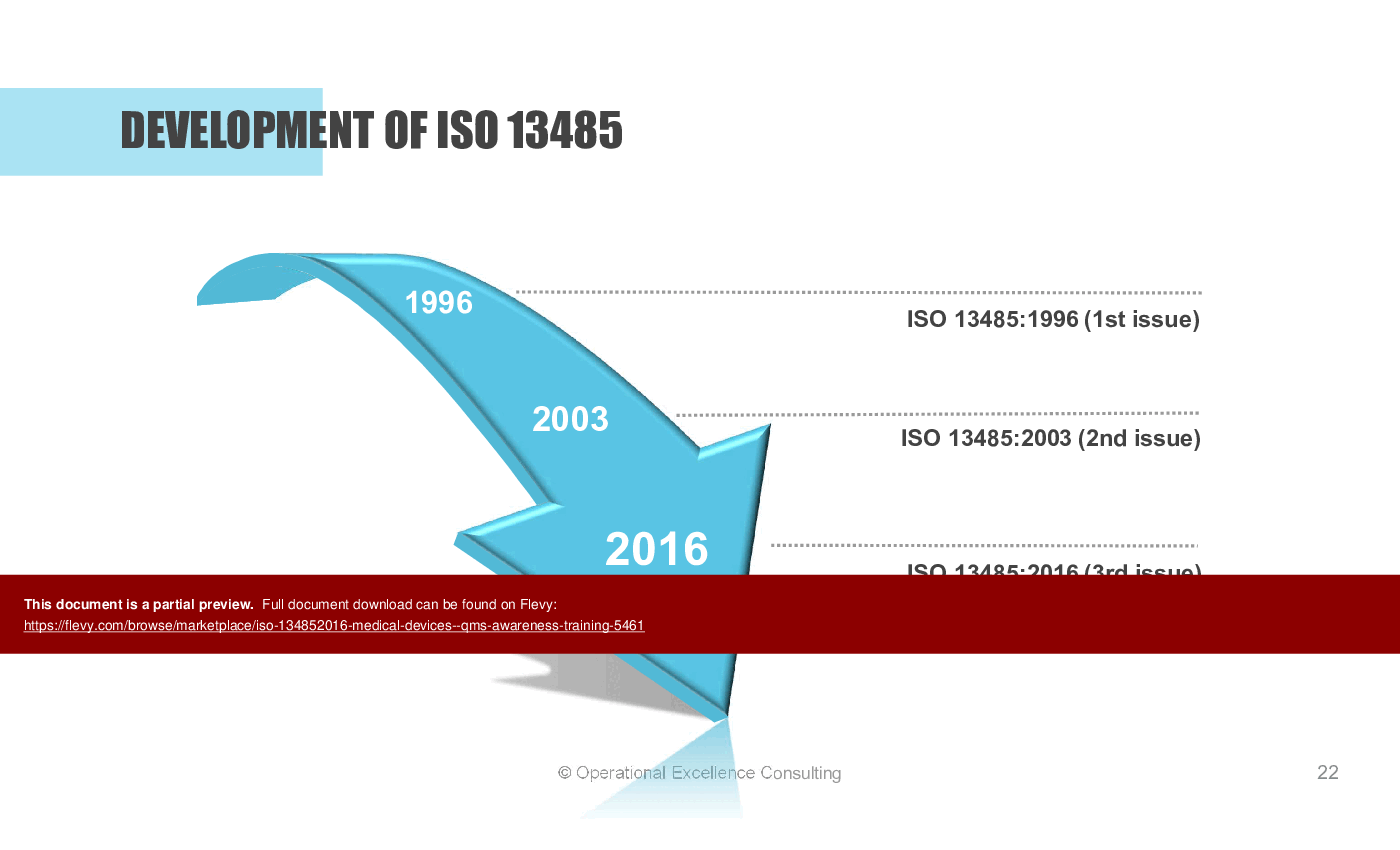
• Development of ISO 13485
• Why Was ISO 13485 Revised?
• Key Improvements to ISO 13485:2016
2. ISO 13485 Structure
• The ISO 13485:2016 Structure
• ISO 13485:2016 Approach is Based on the PDCA Cycle
• ISO 13485:2016 Key Clause Structure (4-8)
• Clause 4: Quality Management System
• Clause 5: Management Responsibility
• Clause 6: Resource Management
• Clause 7: Product Realization
• Clause 8: Measurement, Analysis & Improvement
• Documentation Requirements
• The PDCA Cycle is the Engine of Continuous Improvement
3. ISO 13485 Certification
• Becoming ISO 13485:2016 Certified
• Aligning/Transitioning to ISO 13485:2016
• ISO 13485:2016 Certification Process
• What Does Certification Assure?
4. Audit Approach
• What is a Quality Audit?
• What is an ISO Audit?
• What Are Audits Used For?
• Types of Quality Audits
• Internal Quality Audit
• Principles of Auditing
• Audit Focus
• Audit Approach
• Audit Emphasis
• Document Review
• Audit Findings
5. Handling an Audit Session
• Rights of Auditee
• Rights of Auditor
• How to Handle an Audit Session?
• Auditee's Conduct
• Interacting with Auditors: Do's
• Interacting with Auditors: Don'ts
The training material delves into the structure of ISO 13485:2016, emphasizing the PDCA cycle as the engine of continuous improvement. It also provides insights into the rights and responsibilities of auditors and auditees, ensuring a comprehensive understanding of the audit process.
Got a question about the product? Email us at support@flevy.com or ask the author directly by using the "Ask the Author a Question" form. If you cannot view the preview above this document description, go here to view the large preview instead.
MARCUS OVERVIEW
This synopsis was written by Marcus [?] based on the analysis of the full 67-slide presentation.
Executive Summary
This ISO 13485:2016 Medical Devices Quality Management Systems training presentation is crafted to elevate awareness of ISO 13485 standards and enhance compliance within organizations involved in the medical device lifecycle. Developed by an experienced Quality Manager with a background in global industry leaders, this presentation provides a structured approach to understanding ISO 13485, focusing on audit readiness, risk management, and effective quality management systems. By utilizing this training, organizations can ensure they meet customer and regulatory requirements, ultimately improving customer satisfaction and operational efficiency.
Who This Is For and When to Use
• Quality Assurance Managers in the medical devices sector
• Regulatory Affairs Specialists responsible for compliance
• Internal Auditors conducting ISO audits
• Senior Management overseeing quality management systems
• Training Coordinators developing internal training programs
Best-fit moments to use this deck:
• During onboarding sessions for new employees in quality management
• As part of continuous professional development for quality teams
• Before scheduled ISO audits to prepare staff and processes
• In workshops aimed at improving compliance and quality practices
Learning Objectives
• Define the key elements of ISO 13485 and its significance in the medical device industry
• Describe the ISO 13485:2016 certification process and its requirements
• Identify the structure and components of a quality management system (QMS)
• Explain the audit approach and effective strategies for handling audit sessions
• Gain insights into the importance of risk management in quality systems
• Develop skills to improve customer satisfaction through effective QMS implementation
Table of Contents
• Overview of ISO 13485 (page 5)
• ISO 13485:2016 Structure (page 25)
• ISO 13485:2016 Certification Process (page 32)
• Audit Approach (page 38)
• Handling an Audit Session (page 52)
Primary Topics Covered
• ISO 13485 Overview - An introduction to ISO 13485, detailing its role in establishing quality management systems for medical devices and its focus on regulatory compliance.
• ISO 13485 Structure - A breakdown of the standard's structure, including key clauses that outline the requirements for effective quality management.
• Certification Process - An explanation of the steps involved in achieving ISO 13485 certification, including internal audits and external assessments.
• Audit Approach - Insights into the systematic process of conducting audits to evaluate compliance with ISO standards and improve quality management practices.
• Handling Audit Sessions - Strategies for effectively managing audit sessions, including rights of auditors and auditees, and best practices for interaction.
Deliverables, Templates, and Tools
• ISO 13485 compliance checklist template for internal audits
• Audit preparation guide for staff training
• QMS documentation framework to align with ISO standards
• Risk management assessment tool for quality processes
• Training materials for ISO 13485 awareness sessions
Slide Highlights
• Overview slide detailing the significance of ISO 13485 in the medical device industry
• Structure slide outlining the key clauses of ISO 13485:2016
• Certification process flowchart illustrating the steps to achieve ISO 13485 certification
• Audit approach slide emphasizing the importance of systematic evaluation
• Handling audit session tips slide providing practical advice for auditees
Potential Workshop Agenda
ISO 13485 Overview Session (60 minutes)
• Introduce ISO 13485 and its relevance to the medical device industry
• Discuss the structure and key clauses of the standard
• Review the certification process and requirements
Audit Preparation Workshop (90 minutes)
• Explain the audit approach and types of audits
• Conduct a mock audit to practice handling audit sessions
• Discuss common audit findings and how to address them
Customization Guidance
• Tailor the presentation to include specific organizational policies and procedures related to ISO 13485
• Adjust examples and case studies to reflect the organization's operational context
• Incorporate internal audit results to highlight areas for improvement
Secondary Topics Covered
• Importance of continual improvement in quality management systems
• Overview of regulatory requirements impacting ISO 13485 compliance
• Best practices for risk management in the medical device sector
• Strategies for enhancing customer satisfaction through quality assurance
Topic FAQ
What are the main clauses of ISO 13485:2016 and what do they cover?
ISO 13485:2016 organizes requirements into key clauses focused on QMS fundamentals: Clause 4 (Quality Management System), Clause 5 (Management Responsibility), Clause 6 (Resource Management), Clause 7 (Product Realization), and Clause 8 (Measurement, Analysis & Improvement), collectively addressing documentation, responsibilities, processes, and continual improvement under Clauses 4–8.How does the ISO 13485 certification process typically proceed for a medical device firm?
Certification involves implementing a documented QMS, conducting internal audits, selecting an accredited certification body, and undergoing external assessment to confirm conformity. Organizations commonly prepare with internal audits and documentation alignment; the product’s certification process flowchart explains these steps and appears on page 32.Which types of audits are relevant under ISO 13485 and how are they classified?
Audits relevant to ISO 13485 include internal audits (conducted by the organization), second-party audits (supplier or customer audits), and third-party audits (external certification audits performed by an independent body), reflecting the document’s distinction among internal, second-party, and third-party audit types.What practical steps help staff prepare for an ISO 13485 audit session?
Effective preparation combines internal audits, targeted staff training on ISO requirements, document reviews to ensure evidence of compliance, and practicing audit interactions. The training presentation provides an audit preparation guide and handling-audit-session tips to support these activities and an audit preparation guide is included.What documentation should an ISO 13485 QMS include to demonstrate compliance?
QMS documentation should include documented policies, procedures, work instructions where needed, records demonstrating process execution, and evidence of compliance with applicable ISO requirements. The presentation’s documentation guidance emphasizes policies, procedures, records, and evidence of compliance as core QMS documentation elements.How is risk management integrated into ISO 13485 compliance activities?
Risk management under ISO 13485 is used to identify, assess, and mitigate risks related to medical device safety and regulatory compliance throughout the product lifecycle. The training highlights the role of risk management and provides a risk management assessment tool to support identification and mitigation activities.What is the cost versus value argument for purchasing ISO 13485 training templates instead of building them internally?
Buyers should weigh upfront cost against time saved and faster audit readiness; reusable templates can reduce design time and align materials to standard requirements. The deck supplies practical templates—an ISO 13485 compliance checklist, QMS documentation framework, and a risk management assessment tool—to quantify near-term value.If I must train new hires on ISO 13485, what core topics should the session cover?
New-hire ISO 13485 training should cover the standard’s purpose and benefits, the PDCA model, the clause structure (Clauses 4–8), the certification process, audit approach and conduct, and practical handling of audit sessions; these core topics are organized in the presentation’s Table of Contents and overview slides.Document FAQ
These are questions addressed within this presentation.
What is ISO 13485?
ISO 13485 is an international standard that specifies requirements for a quality management system in the medical device industry, focusing on meeting customer and regulatory requirements.
Who should use this presentation?
This presentation is designed for quality assurance professionals, regulatory affairs specialists, and anyone involved in the medical device lifecycle.
How does the certification process work?
The certification process involves implementing a QMS, conducting internal audits, selecting a certification body, and undergoing external audits to confirm compliance with ISO 13485.
What are the benefits of ISO 13485 certification?
Certification enhances credibility, demonstrates compliance with regulatory requirements, and can improve customer satisfaction and operational efficiency.
What types of audits are there?
Audits can be classified as internal audits, second-party audits (supplier audits), and third-party audits (certification audits).
How can organizations prepare for an ISO audit?
Organizations can prepare by conducting internal audits, training staff on ISO requirements, and reviewing documentation to ensure compliance.
What is the role of risk management in ISO 13485?
Risk management is essential for identifying, assessing, and mitigating risks associated with medical devices, ensuring safety and compliance.
What should be included in QMS documentation?
QMS documentation should include policies, procedures, records, and evidence of compliance with ISO 13485 requirements.
Glossary
• ISO 13485 - International standard for quality management systems in the medical devices industry.
• Quality Management System (QMS) - A structured system for managing an organization's processes to meet stakeholder expectations.
• Certification - The formal recognition that an organization meets the requirements of a specific standard.
• Audit - A systematic examination of a quality system to determine compliance with standards.
• Risk Management - The process of identifying, assessing, and mitigating risks associated with medical devices.
• Nonconformity - A failure to meet a requirement of the standard or the organization's internal processes.
• Internal Audit - An audit conducted within an organization to assess compliance with its own policies and standards.
• External Audit - An audit performed by an independent body to evaluate compliance with ISO standards.
• Continual Improvement - Ongoing efforts to enhance products, services, or processes.
• Regulatory Compliance - Adherence to laws, regulations, and guidelines relevant to the medical device industry.
• Documentation Requirements - Specifications for maintaining records and procedures as per ISO standards.
• Management Responsibility - The obligation of top management to ensure the effectiveness of the QMS.
• Product Realization - The process of planning and executing the production of medical devices.
• Measurement, Analysis & Improvement - The processes involved in monitoring and enhancing the QMS performance.
• Customer Focus - The principle of prioritizing customer needs and satisfaction in quality management.
• Accreditation - The formal recognition that a certification body operates according to international standards.
• Stakeholders - Individuals or groups with an interest in the organization's performance and outcomes.
• Compliance Audit - An audit that evaluates adherence to regulatory and standard requirements.
• Nonconformance Report - A document that outlines deviations from established standards or procedures.
• Corrective Action - Steps taken to eliminate the causes of nonconformities to prevent recurrence.
• Preventive Action - Measures implemented to eliminate potential causes of nonconformities.
• Training Needs Analysis - The process of identifying training requirements for staff to meet ISO standards.
Source: Best Practices in Quality Management, Healthcare, ISO 13485 PowerPoint Slides: ISO 13485:2016 (Medical Devices - QMS) Awareness Training PowerPoint (PPTX) Presentation Slide Deck, Operational Excellence Consulting