100+ Biotech / Pharma Business SOPs (Excel XLSX)
Excel (XLSX)
BENEFITS OF THIS EXCEL DOCUMENT
- Offers a comprehensive roadmap for implementing best practices in regulatory compliance across all stages of your biotech and pharma operations.
- Delivers a structured approach to managing clinical trials, ensuring consistent adherence to industry standards and minimizing compliance risks.
- Equips your organization with a set of proven, customizable SOPs that streamline manufacturing, enhance product quality, and ensure operational excellence.
HEALTHCARE EXCEL DESCRIPTION
Curated by McKinsey-trained Executives
🚀 Unlock the Ultimate Biotech/Pharma SOP Library – 100+ Critical SOP Templates for Your Business
Transform your Biotech and Pharma operations with our 100+ SOP Library—an all-in-one Excel template designed to streamline your business processes, ensure regulatory compliance, and reduce operational risks. Whether you're in Regulatory Compliance, Research & Development, Manufacturing, Pharmacovigilance, Clinical Operations, or Risk Management, this comprehensive library is your key to success.
Save time. Minimize risks. Maximize compliance.
Why Choose the 100+ Biotech/Pharma SOP Library?
• Pre-Built, Ready-to-Use Templates: 100+ SOP templates across 15 clusters, designed for quick implementation.
• Streamline Operations: From R&D to manufacturing to distribution, optimize workflows, reduce errors, and speed up processes.
• Perfect for All Business Sizes: Whether you're a startup, mid-sized company, or a global enterprise, these SOPs grow with your needs.
• Risk Mitigation: Reduce the likelihood of compliance violations, production delays, and legal issues with proven, structured processes.
100+ Biotech/Pharma SOPs – Fully Detailed & Ready for Use
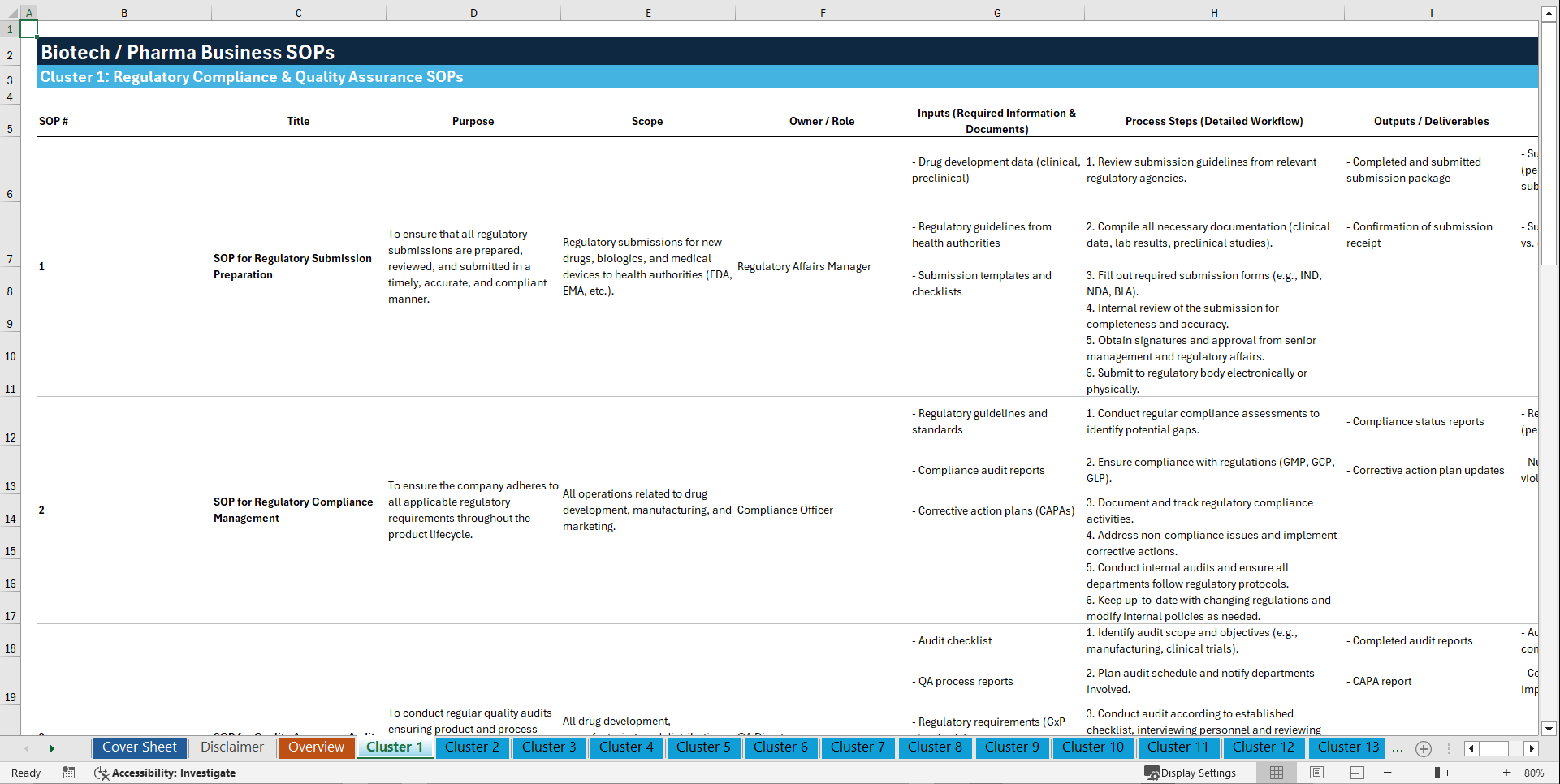
Cluster 1: Regulatory Compliance & Quality Assurance
1. SOP for Regulatory Submission Preparation
2. SOP for Regulatory Compliance Management
3. SOP for Quality Assurance Audits
4. SOP for Document Control
5. SOP for Good Manufacturing Practices (GMP) Compliance
6. SOP for Drug Approval Process
7. SOP for Investigational New Drug (IND) Applications
8. SOP for Quality Control Testing
9. SOP for Regulatory Inspection Preparation
10. SOP for Risk Management and Assessment
Cluster 2: Research & Development
1. SOP for Clinical Trial Protocol Design
2. SOP for Laboratory Equipment Calibration
3. SOP for Good Clinical Practice (GCP) Compliance
4. SOP for Preclinical Research Documentation
5. SOP for In vitro Studies
6. SOP for In vivo Animal Research
7. SOP for Investigator Initiated Trials (IITs)
8. SOP for Protocol Deviation Management
9. SOP for Data Integrity in Clinical Trials
10. SOP for Investigational Product Development
Cluster 3: Manufacturing & Production
1. SOP for Production Batch Record Review
2. SOP for Sterility Testing of Biopharmaceuticals
3. SOP for Manufacturing Process Validation
4. SOP for Cleaning and Sanitization of Equipment
5. SOP for Control of Raw Materials
6. SOP for Equipment Maintenance
7. SOP for Bioreactor Setup and Operation
8. SOP for Labeling and Packaging of Pharmaceutical Products
9. SOP for Stability Testing of Drug Products
10. SOP for Handling of Expired Materials
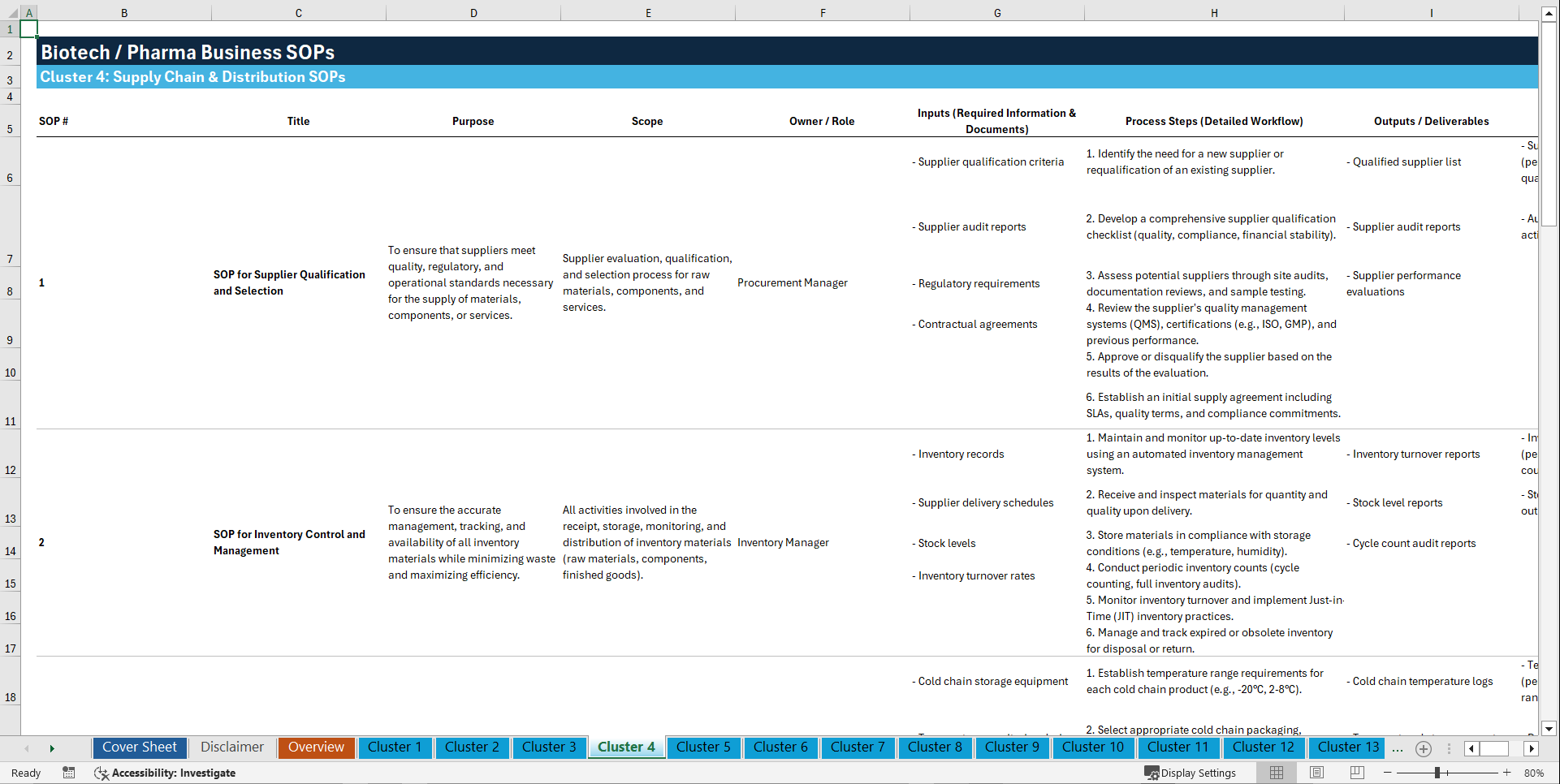
Cluster 4: Supply Chain & Distribution
1. SOP for Supplier Qualification and Selection
2. SOP for Inventory Control and Management
3. SOP for Cold Chain Logistics
4. SOP for Batch Release and Distribution
5. SOP for Receiving and Inspecting Incoming Materials
6. SOP for Warehouse Management
7. SOP for Shipping and Handling of Biological Products
8. SOP for Distribution of Controlled Substances
9. SOP for Documentation and Traceability in the Supply Chain
10. SOP for Expiry Management
Cluster 5: Pharmacovigilance & Safety
1. SOP for Adverse Event Reporting
2. SOP for Safety Data Management
3. SOP for Signal Detection and Risk Assessment
4. SOP for Post-Marketing Surveillance
5. SOP for Periodic Safety Update Reports (PSUR)
6. SOP for Pharmacovigilance Audits
7. SOP for Safety Review Committee Operations
8. SOP for Risk Mitigation Strategies
9. SOP for Clinical Trial Safety Monitoring
10. SOP for Handling Serious Adverse Events (SAE)
Cluster 6: Clinical Operations
1. SOP for Site Selection and Initiation
2. SOP for Investigator Recruitment and Training
3. SOP for Informed Consent Process
4. SOP for Clinical Data Management
5. SOP for Electronic Data Capture (EDC) System Usage
6. SOP for Clinical Trial Monitoring
7. SOP for Trial Master File (TMF) Management
8. SOP for Clinical Trial Close-out Procedures
9. SOP for Patient Enrollment and Retention
10. SOP for Randomization and Blinding
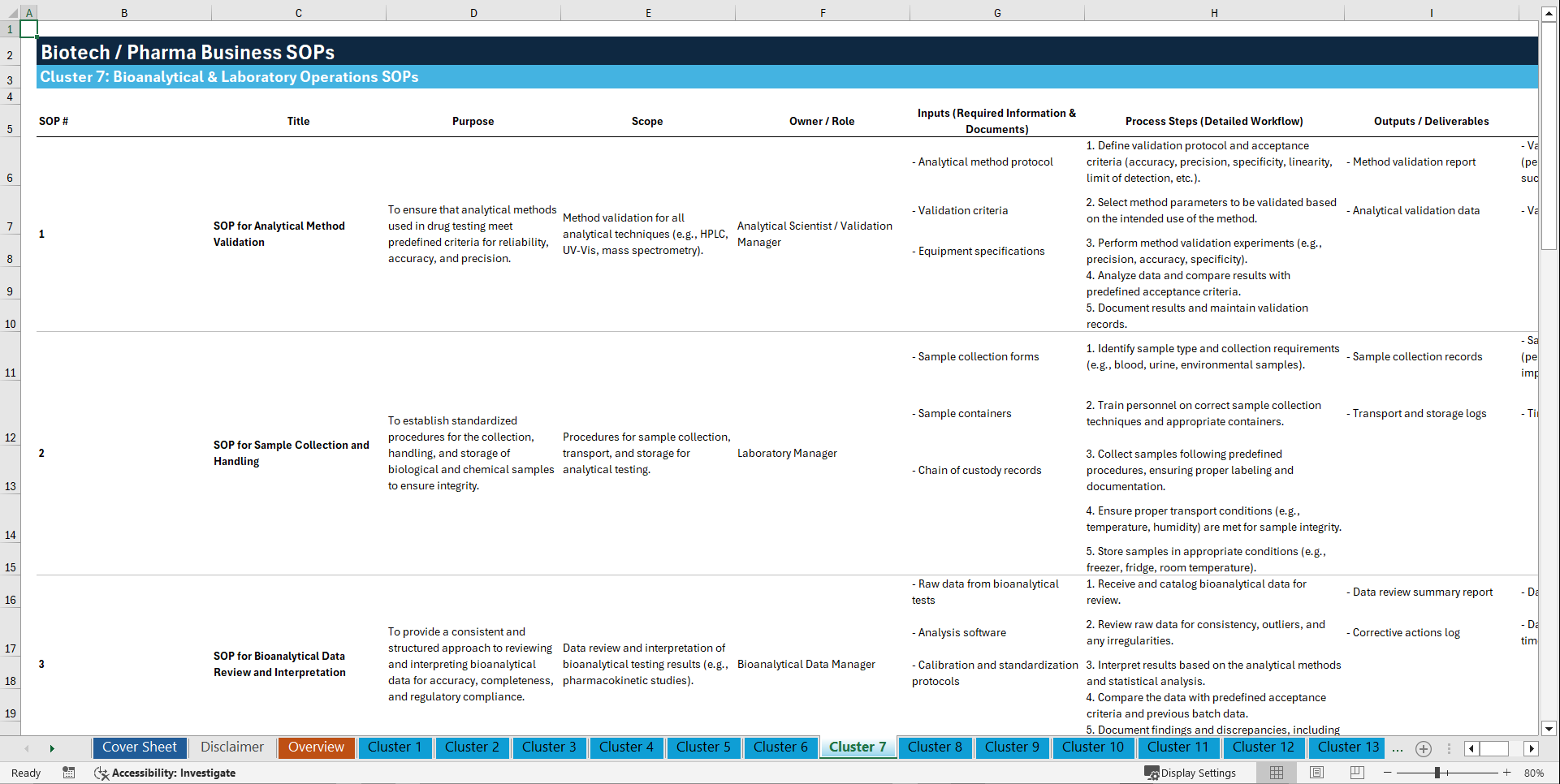
Cluster 7: Bioanalytical & Laboratory Operations
1. SOP for Analytical Method Validation
2. SOP for Sample Collection and Handling
3. SOP for Bioanalytical Data Review and Interpretation
4. SOP for Instrument Calibration and Maintenance
5. SOP for Bioassay Development
6. SOP for Stability Studies of Active Pharmaceutical Ingredients (APIs)
7. SOP for Analytical Laboratory Safety
8. SOP for Data Integrity and Retention
9. SOP for GLP Compliance in Bioanalytical Testing
10. SOP for Contamination Control in Laboratories
Cluster 8: Finance & Contract Management
1. SOP for Contract Development and Negotiation
2. SOP for Budgeting and Financial Planning for Clinical Trials
3. SOP for Cost Control in Drug Development
4. SOP for Grant Management and Funding Allocation
5. SOP for Payment Disbursement to Vendors and Contractors
6. SOP for Invoice and Expense Tracking
7. SOP for Financial Reporting and Audit Preparation
8. SOP for License and Royalties Management
9. SOP for Revenue Recognition in Pharma Sales
10. SOP for Tax Compliance and Reporting
Cluster 9: IT & Data Management
1. SOP for Data Security and Privacy Management
2. SOP for Electronic Signature Use and Validation
3. SOP for Software Validation and Qualification
4. SOP for Electronic Data Archiving
5. SOP for IT Incident Management and Response
6. SOP for Data Backup and Disaster Recovery
7. SOP for Managing Clinical Trial Data in Cloud Systems
8. SOP for Digital Platform Integration
9. SOP for Cybersecurity Risk Management
10. SOP for Clinical Trial Management System (CTMS) Usage
Cluster 10: Human Resources & Training
1. SOP for Staff Recruitment and Hiring Process
2. SOP for Employee Onboarding and Training
3. SOP for Employee Health and Safety Compliance
4. SOP for Performance Management and Evaluation
5. SOP for Maintaining Employee Records
6. SOP for Continuing Education in Compliance
7. SOP for Staff Role Assignments in Clinical Trials
8. SOP for External Training and Certifications
9. SOP for Conflict Resolution in the Workplace
10. SOP for Employee Termination and Exit Interviews
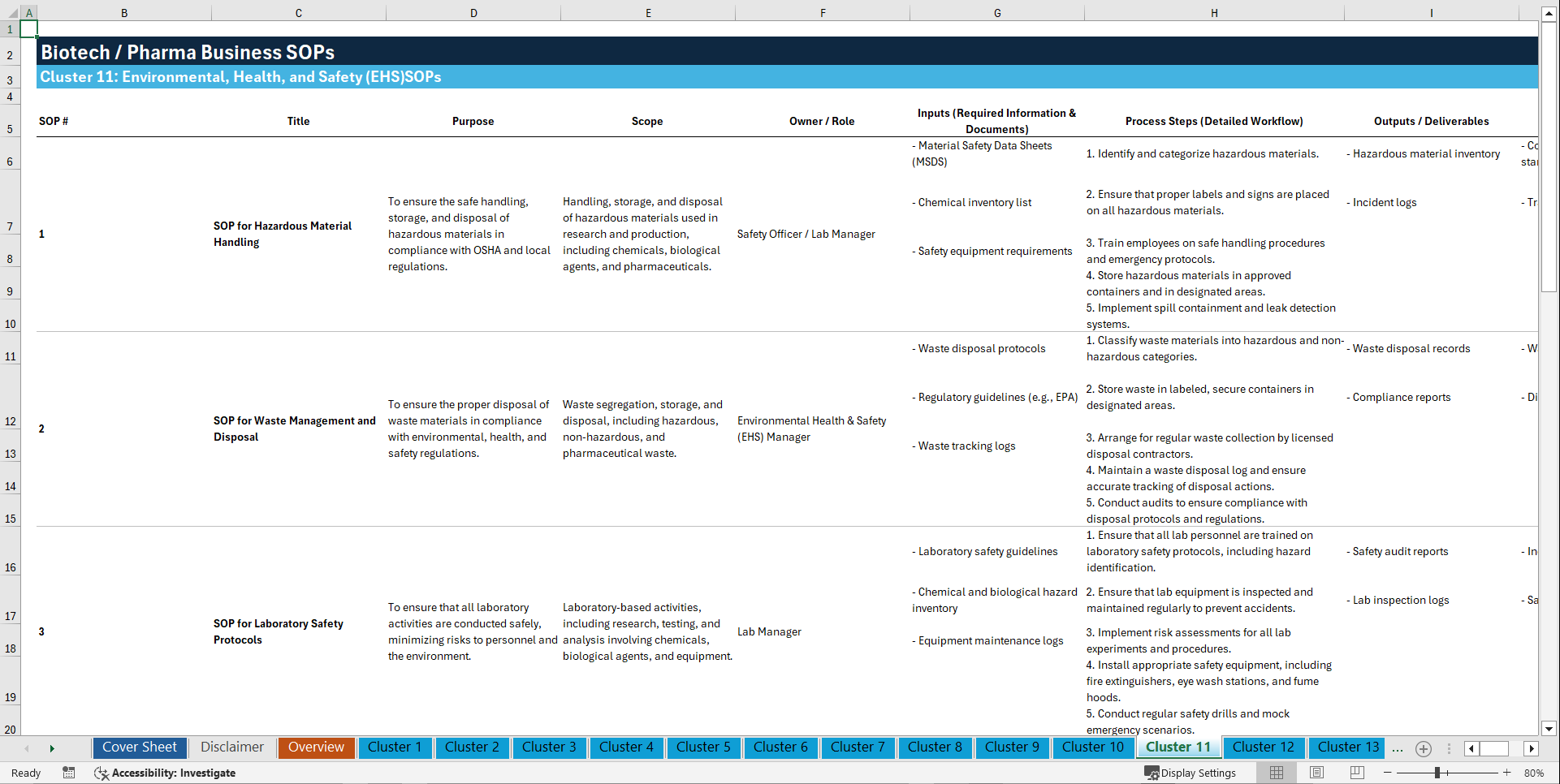
Cluster 11: Environmental, Health, and Safety (EHS)
1. SOP for Hazardous Material Handling
2. SOP for Waste Management and Disposal
3. SOP for Laboratory Safety Protocols
4. SOP for Workplace Safety Audits
5. SOP for Chemical Spill Response and Cleanup
6. SOP for Personal Protective Equipment (PPE) Use
7. SOP for Environmental Impact Assessments
8. SOP for Fire Safety and Evacuation Procedures
9. SOP for Employee Exposure Monitoring
10. SOP for Occupational Health Monitoring
Cluster 12: Commercialization & Market Access
1. SOP for Market Research and Competitive Intelligence
2. SOP for Product Launch Strategy
3. SOP for Pricing and Reimbursement Strategies
4. SOP for Commercial Sales and Distribution Network
5. SOP for Post-Launch Market Surveillance
6. SOP for Key Opinion Leader (KOL) Engagement
7. SOP for Product Life Cycle Management
8. SOP for Forecasting Drug Demand and Supply
9. SOP for Market Access Strategy in Global Markets
10. SOP for Regulatory Compliance in Marketing Materials
Cluster 13: Clinical Trial Ethics & Patient Engagement
1. SOP for Ethical Committee Submission and Approval
2. SOP for Patient Informed Consent and Understanding
3. SOP for Handling Patient Confidentiality and Data Protection
4. SOP for Handling Vulnerable Populations in Clinical Trials
5. SOP for Participant Safety Monitoring
6. SOP for Patient Recruitment and Screening Criteria
7. SOP for Management of Clinical Trial Complaints
8. SOP for Clinical Trial Non-Compliance
9. SOP for Patient Retention Strategies
10. SOP for Ensuring Scientific Integrity in Trials
Cluster 14: Risk Management & Crisis Response
1. SOP for Crisis Management and Communication
2. SOP for Risk Assessment and Mitigation in Clinical Trials
3. SOP for Business Continuity and Contingency Planning
4. SOP for Post-Market Risk Management Plans
5. SOP for Clinical Trial Emergency Response
6. SOP for Root Cause Analysis in Quality Issues
7. SOP for Crisis Simulation and Drills
8. SOP for Regulatory Agency Communication During Crisis
9. SOP for Contingency Plans in Drug Manufacturing
10. SOP for Risk-Based Monitoring in Clinical Trials
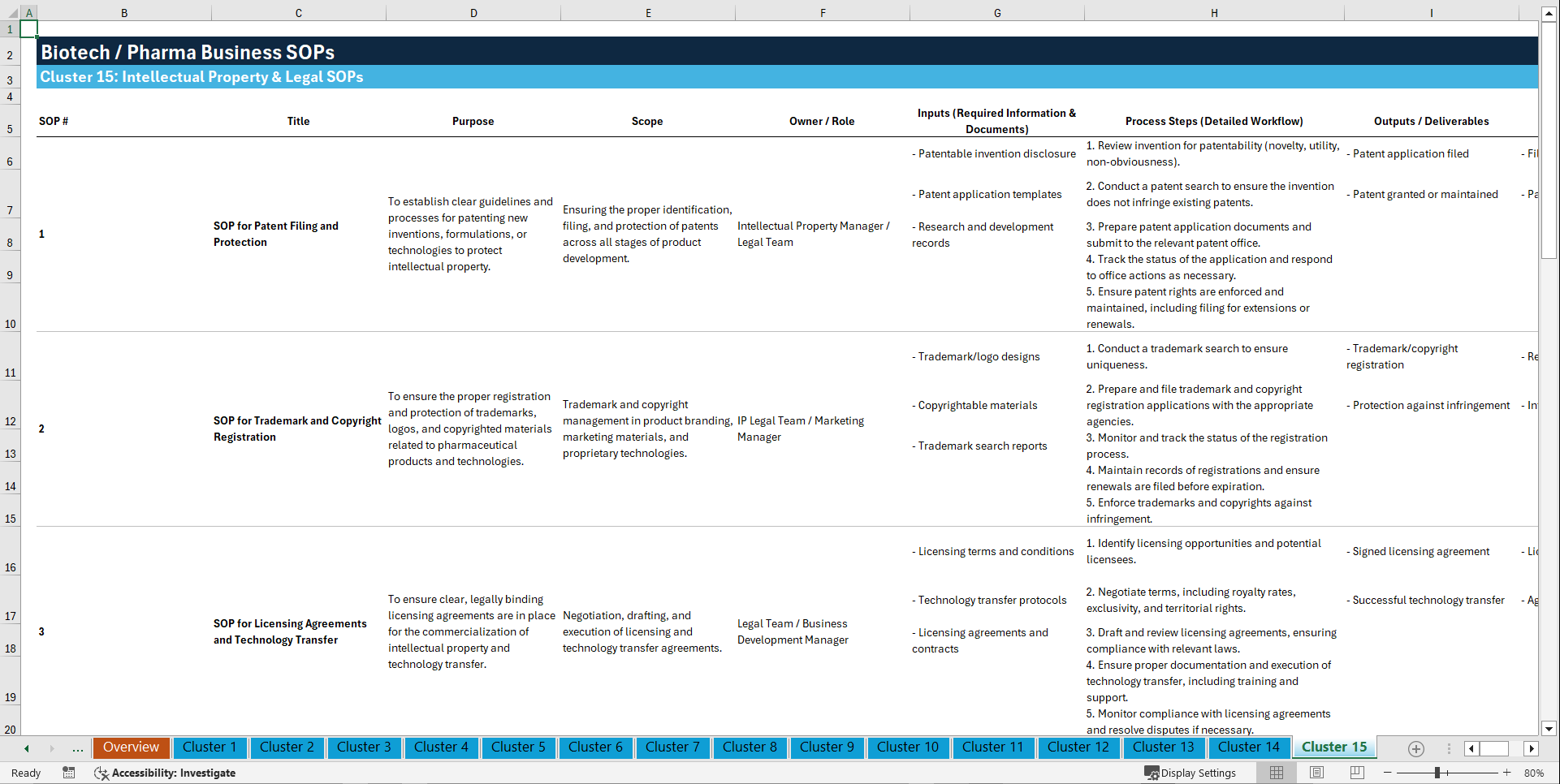
Cluster 15: Intellectual Property & Legal
1. SOP for Patent Filing and Protection
2. SOP for Trademark and Copyright Registration
3. SOP for Licensing Agreements and Technology Transfer
4. SOP for Legal Compliance in Drug Marketing
5. SOP for Handling Patent Disputes
6. SOP for Non-Disclosure Agreements (NDAs)
7. SOP for Compliance with Anti-Kickback Statutes
8. SOP for Clinical Trial Liability Management
9. SOP for Import/Export Compliance
10. SOP for Compliance with International Laws and Regulations
Why This SOP Library is a MUST-HAVE:
• 100+ Proven SOPs: Ready to use, customize, and implement across your operations.
• Comprehensive Coverage: SOPs cover everything from Regulatory Compliance to Pharmacovigilance, Clinical Trials, and Market Access.
• Fast, Efficient, and Scalable: Accelerate time-to-market with standardized processes that ensure quality and compliance.
Get Your SOP Library Today!
The 100+ Biotech/Pharma SOP Library is your essential tool for achieving operational excellence.
Ready to streamline your operations and boost your business?
#purchase and unlock the full library of SOP templates now!
Optimize Your Biotech/Pharma Business – Compliance. Efficiency. Growth.
Key Words:
Strategy & Transformation, Growth Strategy, Strategic Planning, Strategy Frameworks, Innovation Management, Pricing Strategy, Core Competencies, Strategy Development, Business Transformation, Marketing Plan Development, Product Strategy, Breakout Strategy, Competitive Advantage, Mission, Vision, Values, Strategy Deployment & Execution, Innovation, Vision Statement, Core Competencies Analysis, Corporate Strategy, Product Launch Strategy, BMI, Blue Ocean Strategy, Breakthrough Strategy, Business Model Innovation, Business Strategy Example, Corporate Transformation, Critical Success Factors, Customer Segmentation, Customer Value Proposition, Distinctive Capabilities, Enterprise Performance Management, KPI, Key Performance Indicators, Market Analysis, Market Entry Example, Market Entry Plan, Market Intelligence, Market Research, Market Segmentation, Market Sizing, Marketing, Michael Porter's Value Chain, Organizational Transformation, Performance Management, Performance Measurement, Platform Strategy, Product Go-to-Market Strategy, Reorganization, Restructuring, SWOT, SWOT Analysis, Service 4.0, Service Strategy, Service Transformation, Strategic Analysis, Strategic Plan Example, Strategy Deployment, Strategy Execution, Strategy Frameworks Compilation, Strategy Methodologies, Strategy Report Example, Value Chain, Value Chain Analysis, Value Innovation, Value Proposition, Vision Statement, Corporate Strategy, Business Development, Business plan pdf, business plan, PDF, Business Plan DOC, Business Plan Template, PPT, Market strategy playbook, strategic market planning, competitive analysis tools, market segmentation frameworks, growth strategy templates, product positioning strategy, market execution toolkit, strategic alignment playbook, KPI and OKR frameworks, business growth strategy guide, cross-functional strategy templates, market risk management, market strategy PowerPoint doc, guide, ebook, e-book ,McKinsey Change Playbook, Organizational change management toolkit, Change management frameworks 2025, Influence model for change, Change leadership strategies, Behavioral change in organizations, Change management PowerPoint templates, Transformational leadership in change, supply chain KPIs, supply chain KPI toolkit, supply chain PowerPoint template, logistics KPIs, procurement KPIs, inventory management KPIs, supply chain performance metrics, manufacturing KPIs, supply chain dashboard, supply chain strategy KPIs, reverse logistics KPIs, sustainability KPIs in supply chain, financial supply chain KPIs, warehouse KPIs, digital supply chain KPIs, 1200 KPIs, supply chain scorecard, KPI examples, supply chain templates, Corporate Finance SOPs, Finance SOP Excel Template, CFO Toolkit, Finance Department Procedures, Financial Planning SOPs, Treasury SOPs, Accounts Payable SOPs, Accounts Receivable SOPs, General Ledger SOPs, Accounting Policies Template, Internal Controls SOPs, Finance Process Standardization, Finance Operating Procedures, Finance Department Excel Template, FP&A Process Documentation, Corporate Finance Template, Finance SOP Toolkit, CFO Process Templates, Accounting SOP Package, Tax Compliance SOPs, Financial Risk Management Procedures.
NOTE: Our digital products are sold on an "as is" basis, making returns and refunds unavailable post-download. Please preview and inquire before purchasing. Please contact us before purchasing if you have any questions! This policy aligns with the standard Flevy Terms of Usage.
Got a question about the product? Email us at support@flevy.com or ask the author directly by using the "Ask the Author a Question" form. If you cannot view the preview above this document description, go here to view the large preview instead.
Source: Best Practices in Healthcare, Biotech Excel: 100+ Biotech / Pharma Business SOPs Excel (XLSX) Spreadsheet, SB Consulting