100+ Good Manufacturing Practices (GMP) SOPs (Excel XLSX)
Excel (XLSX)
BENEFITS OF THIS EXCEL DOCUMENT
- Provides a complete, ready-to-use framework for building a fully compliant GMP Quality Management System across all operational areas.
- Enables your organization to standardize processes, strengthen regulatory compliance, and streamline audit readiness with minimal effort.
- Offers a comprehensive foundation for developing, managing, and optimizing GMP procedures tailored to your facility's unique manufacturing environment.
GOOD MANUFACTURING PRACTICE EXCEL DESCRIPTION
Curated by McKinsey-trained Executives
🚀 The Ultimate 100+ GMP SOPs Excel Template Package
If your pharmaceutical, biotech, cosmetic, medical device, nutraceutical, or food manufacturing facility is still struggling with incomplete, outdated, or non-compliant GMP documentation, this is your moment.
Introducing the Most Comprehensive Good Manufacturing Practices (GMP) SOP Package on the Market—a 150-document mega-bundle, professionally structured into a fully editable Excel-based SOP management system.
This pack is specifically engineered for GMP compliance, audit readiness, FDA 21 CFR Part 210/211, EU GMP, ICH Q10, ISO 9001, ISO 22716, WHO GMP, and all major global regulatory frameworks.
Whether you're launching a facility, upgrading compliance standards, preparing for inspections, or expanding your QMS from scratch, this package provides every SOP you need—clearly organized, editable, audit-friendly, and instantly deployable.
🔥 Why This GMP SOP Package Is the #1 Choice for Compliance-Driven Manufacturers
✔ 150 fully editable SOPs covering every core GMP process
✔ Delivered as a complete Excel SOP Management System
✔ Instant download—zero setup required
✔ Designed by GMP specialists and QA professionals
✔ Reduces documentation development time by 90%
✔ Perfect for FDA, EMA, MHRA, PIC/S, WHO and ISO compliance
✔ Ideal for pharma, bioscience, cosmetics, supplements, F&B and more
This package gives you a done-for-you framework to build a world-class Quality Management System, streamline operations, pass audits with confidence, prevent deviations, and ensure full-spectrum GMP control from raw materials to distribution.
If your goal is stronger compliance, reduced risk, and standardized processes across all departments, this is the most valuable GMP toolkit you will ever invest in.
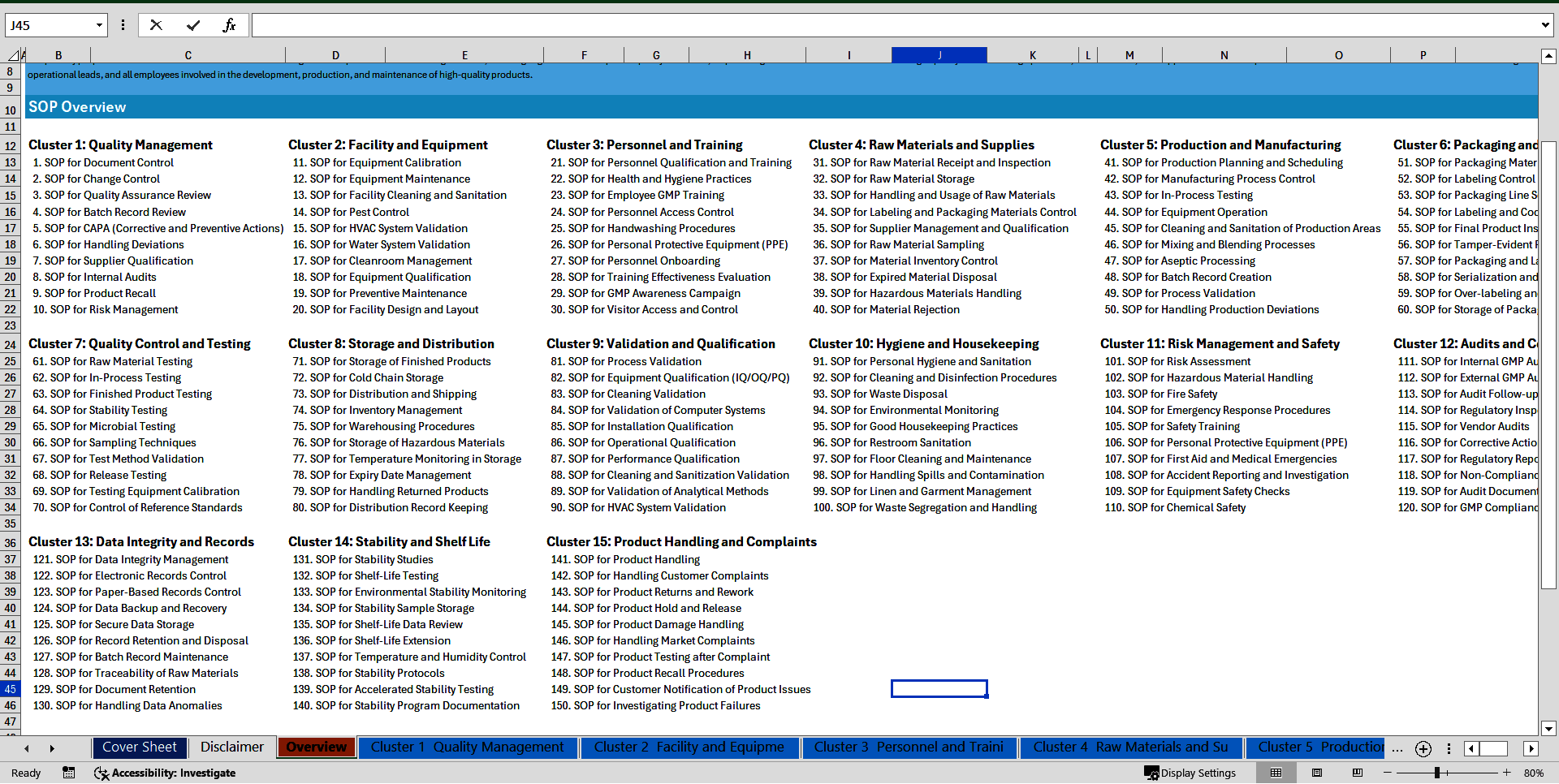
✅ FULL LIST OF ALL 150 GMP SOPs INCLUDED
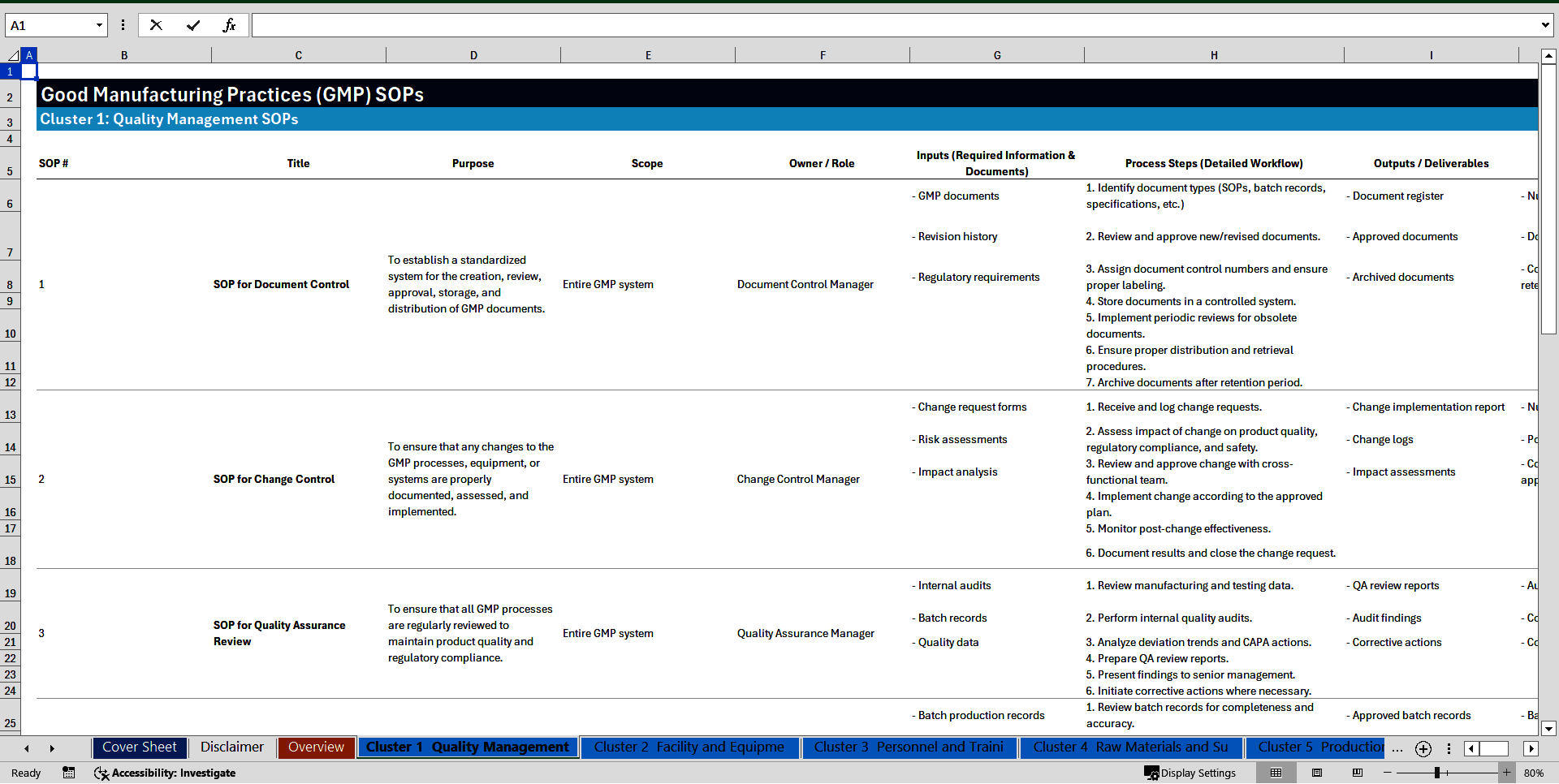
Cluster 1: Quality Management
1. SOP for Document Control
2. SOP for Change Control
3. SOP for Quality Assurance Review
4. SOP for Batch Record Review
5. SOP for CAPA (Corrective and Preventive Actions)
6. SOP for Handling Deviations
7. SOP for Supplier Qualification
8. SOP for Internal Audits
9. SOP for Product Recall
10. SOP for Risk Management
Cluster 2: Facility and Equipment
11. SOP for Equipment Calibration
12. SOP for Equipment Maintenance
13. SOP for Facility Cleaning and Sanitation
14. SOP for Pest Control
15. SOP for HVAC System Validation
16. SOP for Water System Validation
17. SOP for Cleanroom Management
18. SOP for Equipment Qualification
19. SOP for Preventive Maintenance
20. SOP for Facility Design and Layout
Cluster 3: Personnel and Training
21. SOP for Personnel Qualification and Training
22. SOP for Health and Hygiene Practices
23. SOP for Employee GMP Training
24. SOP for Personnel Access Control
25. SOP for Handwashing Procedures
26. SOP for Personal Protective Equipment (PPE)
27. SOP for Personnel Onboarding
28. SOP for Training Effectiveness Evaluation
29. SOP for GMP Awareness Campaign
30. SOP for Visitor Access and Control
Cluster 4: Raw Materials and Supplies
31. SOP for Raw Material Receipt and Inspection
32. SOP for Raw Material Storage
33. SOP for Handling and Usage of Raw Materials
34. SOP for Labeling and Packaging Materials Control
35. SOP for Supplier Management and Qualification
36. SOP for Raw Material Sampling
37. SOP for Material Inventory Control
38. SOP for Expired Material Disposal
39. SOP for Hazardous Materials Handling
40. SOP for Material Rejection
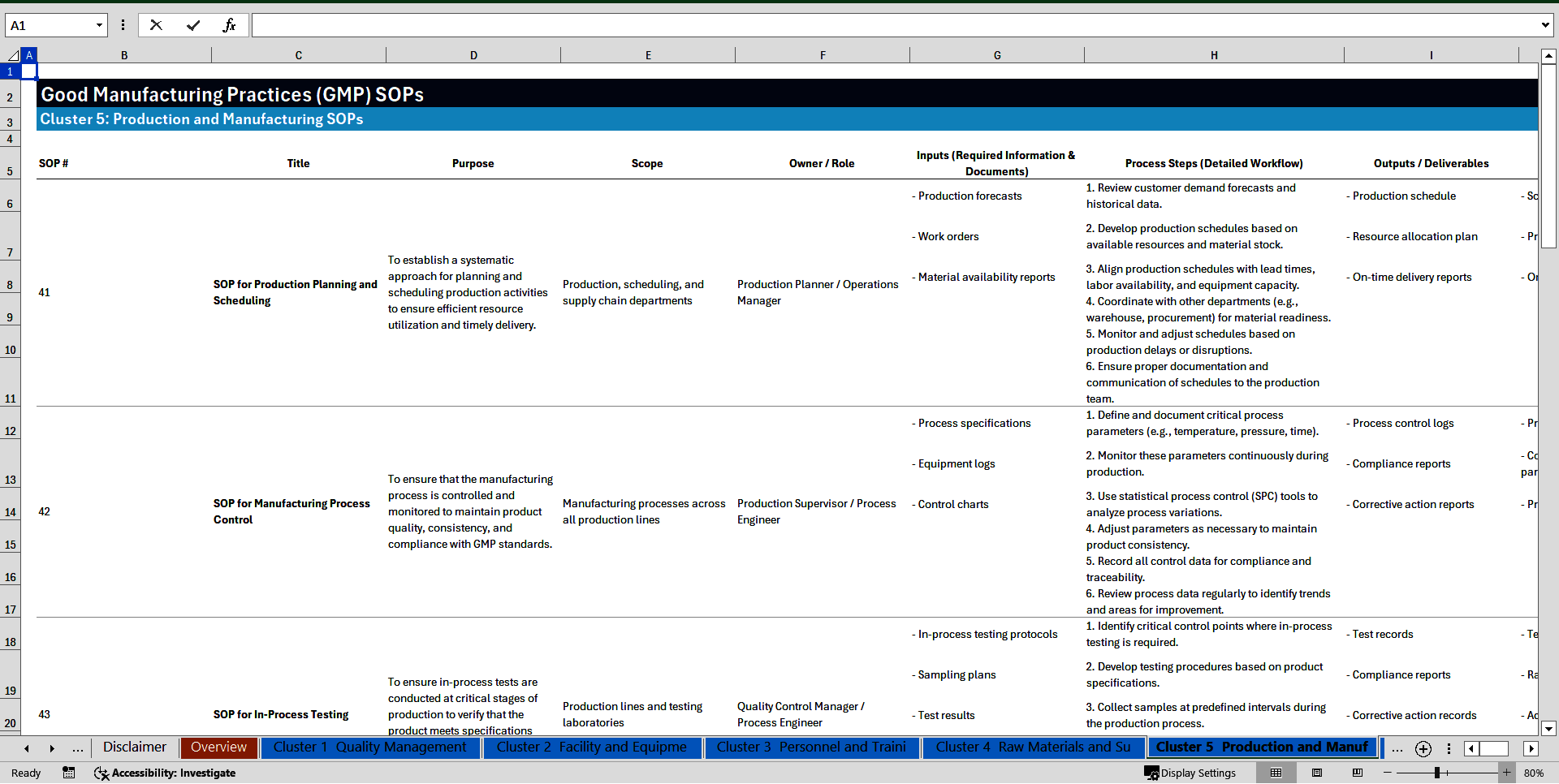
Cluster 5: Production and Manufacturing
41. SOP for Production Planning and Scheduling
42. SOP for Manufacturing Process Control
43. SOP for In-Process Testing
44. SOP for Equipment Operation
45. SOP for Cleaning and Sanitation of Production Areas
46. SOP for Mixing and Blending Processes
47. SOP for Aseptic Processing
48. SOP for Batch Record Creation
49. SOP for Process Validation
50. SOP for Handling Production Deviations
Cluster 6: Packaging and Labeling
51. SOP for Packaging Material Inspection
52. SOP for Labeling Control and Verification
53. SOP for Packaging Line Setup
54. SOP for Labeling and Coding Procedures
55. SOP for Final Product Inspection
56. SOP for Tamper-Evident Packaging
57. SOP for Packaging and Labeling Records
58. SOP for Serialization and Track-and-Trace
59. SOP for Over-labeling and Re-labeling
60. SOP for Storage of Packaged Products
Cluster 7: Quality Control and Testing
61. SOP for Raw Material Testing
62. SOP for In-Process Testing
63. SOP for Finished Product Testing
64. SOP for Stability Testing
65. SOP for Microbial Testing
66. SOP for Sampling Techniques
67. SOP for Test Method Validation
68. SOP for Release Testing
69. SOP for Testing Equipment Calibration
70. SOP for Control of Reference Standards
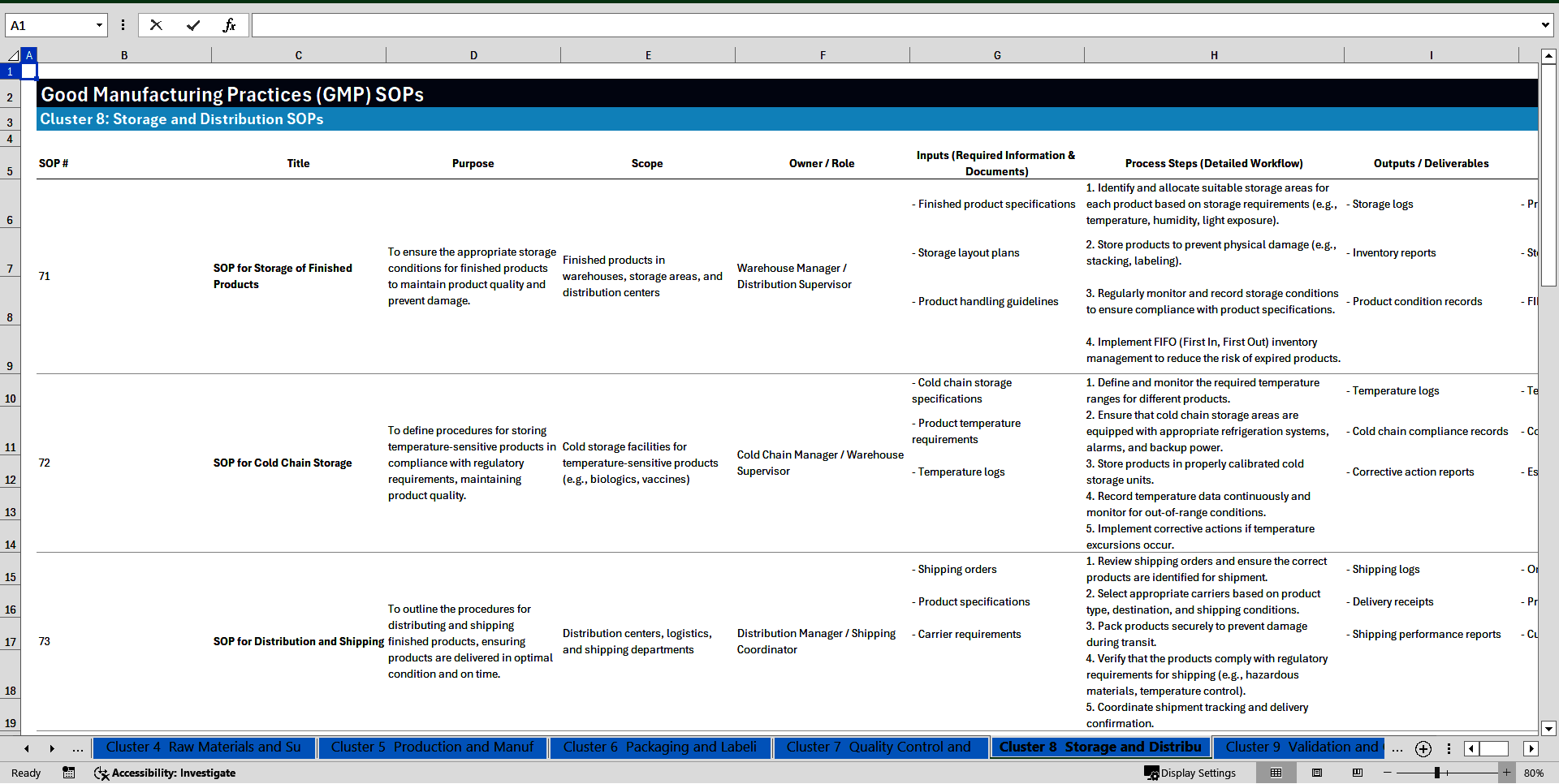
Cluster 8: Storage and Distribution
71. SOP for Storage of Finished Products
72. SOP for Cold Chain Storage
73. SOP for Distribution and Shipping
74. SOP for Inventory Management
75. SOP for Warehousing Procedures
76. SOP for Storage of Hazardous Materials
77. SOP for Temperature Monitoring in Storage
78. SOP for Expiry Date Management
79. SOP for Handling Returned Products
80. SOP for Distribution Record Keeping
Cluster 9: Validation and Qualification
81. SOP for Process Validation
82. SOP for Equipment Qualification (IQ/OQ/PQ)
83. SOP for Cleaning Validation
84. SOP for Validation of Computer Systems
85. SOP for Installation Qualification
86. SOP for Operational Qualification
87. SOP for Performance Qualification
88. SOP for Cleaning and Sanitization Validation
89. SOP for Validation of Analytical Methods
90. SOP for HVAC System Validation
Cluster 10: Hygiene and Housekeeping
91. SOP for Personal Hygiene and Sanitation
92. SOP for Cleaning and Disinfection Procedures
93. SOP for Waste Disposal
94. SOP for Environmental Monitoring
95. SOP for Good Housekeeping Practices
96. SOP for Restroom Sanitation
97. SOP for Floor Cleaning and Maintenance
98. SOP for Handling Spills and Contamination
99. SOP for Linen and Garment Management
100. SOP for Waste Segregation and Handling
Cluster 11: Risk Management and Safety
101. SOP for Risk Assessment
102. SOP for Hazardous Material Handling
103. SOP for Fire Safety
104. SOP for Emergency Response Procedures
105. SOP for Safety Training
106. SOP for Personal Protective Equipment (PPE)
107. SOP for First Aid and Medical Emergencies
108. SOP for Accident Reporting and Investigation
109. SOP for Equipment Safety Checks
110. SOP for Chemical Safety
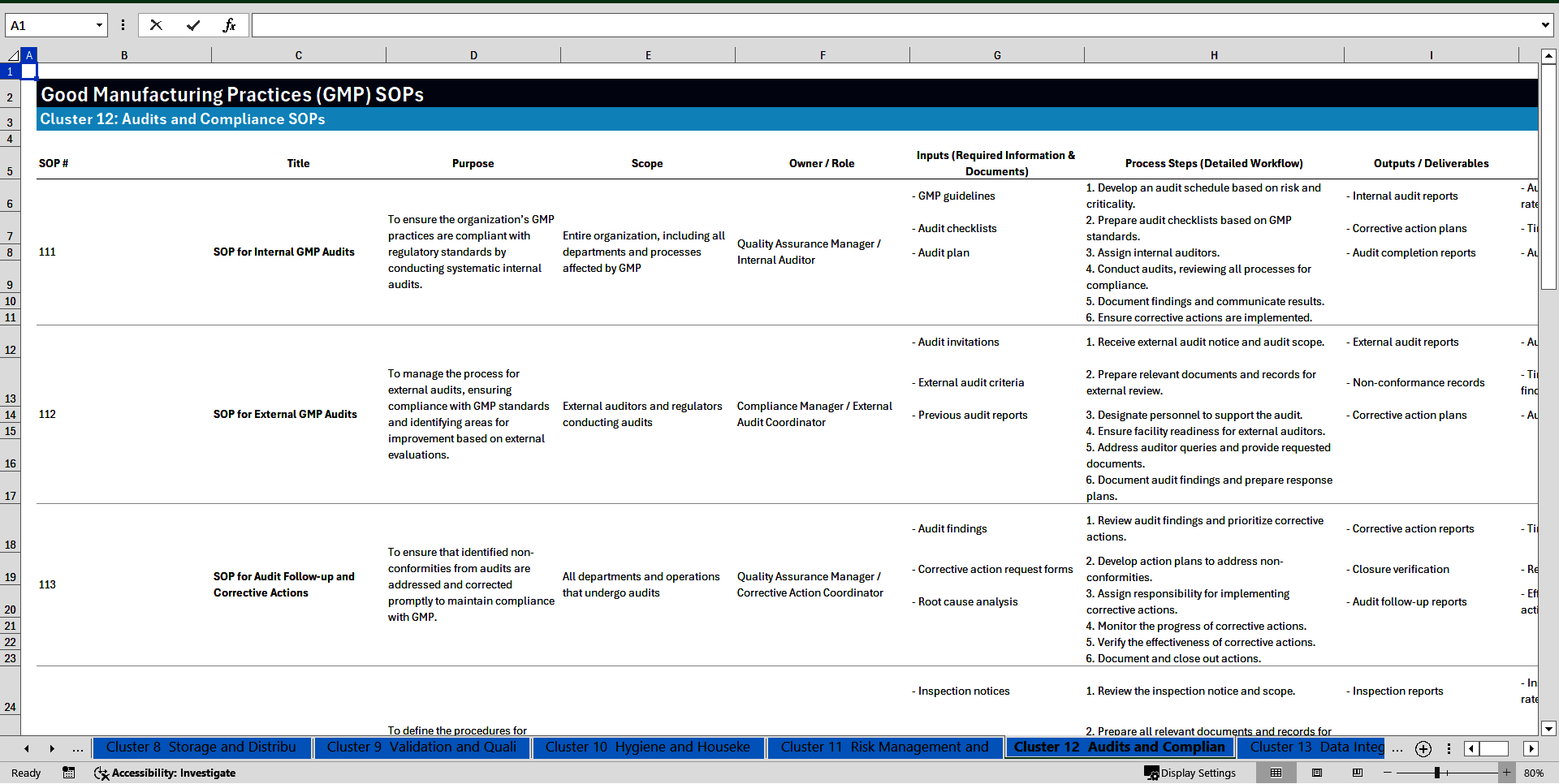
Cluster 12: Audits and Compliance
111. SOP for Internal GMP Audits
112. SOP for External GMP Audits
113. SOP for Audit Follow-up and Corrective Actions
114. SOP for Regulatory Inspections
115. SOP for Vendor Audits
116. SOP for Corrective Action Request Management
117. SOP for Regulatory Reporting
118. SOP for Non-Compliance Management
119. SOP for Audit Documentation and Record Keeping
120. SOP for GMP Compliance Monitoring
Cluster 13: Data Integrity and Records
121. SOP for Data Integrity Management
122. SOP for Electronic Records Control
123. SOP for Paper-Based Records Control
124. SOP for Data Backup and Recovery
125. SOP for Secure Data Storage
126. SOP for Record Retention and Disposal
127. SOP for Batch Record Maintenance
128. SOP for Traceability of Raw Materials
129. SOP for Document Retention
130. SOP for Handling Data Anomalies
Cluster 14: Stability and Shelf Life
131. SOP for Stability Studies
132. SOP for Shelf-Life Testing
133. SOP for Environmental Stability Monitoring
134. SOP for Stability Sample Storage
135. SOP for Shelf-Life Data Review
136. SOP for Shelf-Life Extension
137. SOP for Temperature and Humidity Control
138. SOP for Stability Protocols
139. SOP for Accelerated Stability Testing
140. SOP for Stability Program Documentation
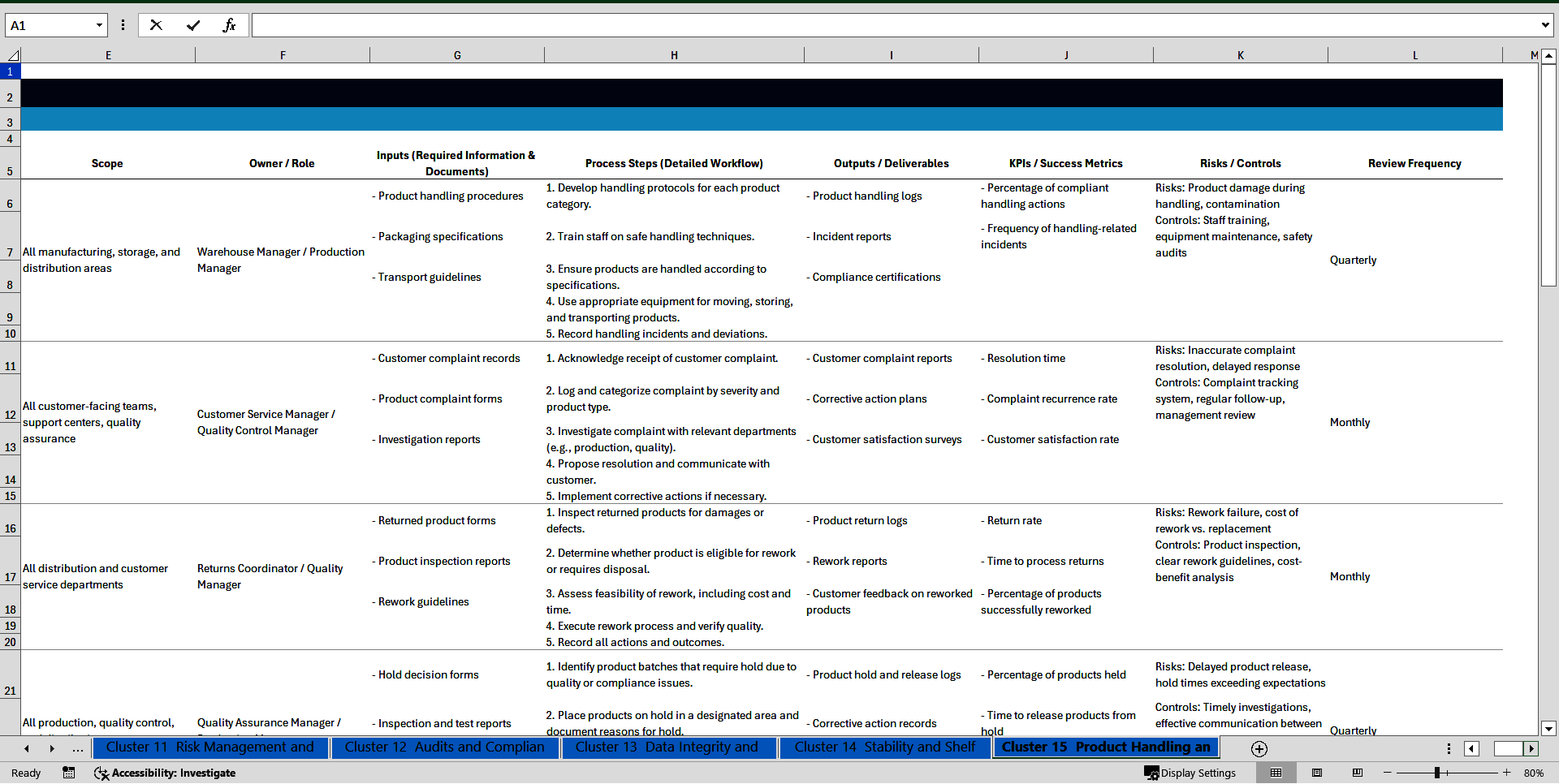
Cluster 15: Product Handling and Complaints
141. SOP for Product Handling
142. SOP for Handling Customer Complaints
143. SOP for Product Returns and Rework
144. SOP for Product Hold and Release
145. SOP for Product Damage Handling
146. SOP for Handling Market Complaints
147. SOP for Product Testing after Complaint
148. SOP for Product Recall Procedures
149. SOP for Customer Notification of Product Issues
150. SOP for Investigating Product Failures
Key Words:
Strategy & Transformation, Growth Strategy, Strategic Planning, Strategy Frameworks, Innovation Management, Pricing Strategy, Core Competencies, Strategy Development, Business Transformation, Marketing Plan Development, Product Strategy, Breakout Strategy, Competitive Advantage, Mission, Vision, Values, Strategy Deployment & Execution, Innovation, Vision Statement, Core Competencies Analysis, Corporate Strategy, Product Launch Strategy, BMI, Blue Ocean Strategy, Breakthrough Strategy, Business Model Innovation, Business Strategy Example, Corporate Transformation, Critical Success Factors, Customer Segmentation, Customer Value Proposition, Distinctive Capabilities, Enterprise Performance Management, KPI, Key Performance Indicators, Market Analysis, Market Entry Example, Market Entry Plan, Market Intelligence, Market Research, Market Segmentation, Market Sizing, Marketing, Michael Porter's Value Chain, Organizational Transformation, Performance Management, Performance Measurement, Platform Strategy, Product Go-to-Market Strategy, Reorganization, Restructuring, SWOT, SWOT Analysis, Service 4.0, Service Strategy, Service Transformation, Strategic Analysis, Strategic Plan Example, Strategy Deployment, Strategy Execution, Strategy Frameworks Compilation, Strategy Methodologies, Strategy Report Example, Value Chain, Value Chain Analysis, Value Innovation, Value Proposition, Vision Statement, Corporate Strategy, Business Development, Busienss plan pdf, business plan, PDF, Biusiness Plan DOC, Bisiness Plan Template, PPT, Market strategy playbook, strategic market planning, competitive analysis tools, market segmentation frameworks, growth strategy templates, product positioning strategy, market execution toolkit, strategic alignment playbook, KPI and OKR frameworks, business growth strategy guide, cross-functional strategy templates, market risk management, market strategy PowerPoint dec, guide, ebook, e-book ,McKinsey Change Playbook, Organizational change management toolkit, Change management frameworks 2025, Influence model for change, Change leadership strategies, Behavioral change in organizations, Change management PowerPoint templates, Transformational leadership in change, supply chain KPIs, supply chain KPI toolkit, supply chain PowerPoint template, logistics KPIs, procurement KPIs, inventory management KPIs, supply chain performance metrics, manufacturing KPIs, supply chain dashboard, supply chain strategy KPIs, reverse logistics KPIs, sustainability KPIs in supply chain, financial supply chain KPIs, warehouse KPIs, digital supply chain KPIs, 1200 KPIs, supply chain scorecard, KPI examples, supply chain templates, Corporate Finance SOPs, Finance SOP Excel Template, CFO Toolkit, Finance Department Procedures, Financial Planning SOPs, Treasury SOPs, Accounts Payable SOPs, Accounts Receivable SOPs, General Ledger SOPs, Accounting Policies Template, Internal Controls SOPs, Finance Process Standardization, Finance Operating Procedures, Finance Department Excel Template, FP&A Process Documentation, Corporate Finance Template, Finance SOP Toolkit, CFO Process Templates, Accounting SOP Package, Tax Compliance SOPs, Financial Risk Management Procedures.
NOTE: Our digital products are sold on an "as is" basis, making returns and refunds unavailable post-download. Please preview and inquire before purchasing. Please contact us before purchasing if you have any questions! This policy aligns with the standard Flevy Terms of Usage.
Got a question about the product? Email us at support@flevy.com or ask the author directly by using the "Ask the Author a Question" form. If you cannot view the preview above this document description, go here to view the large preview instead.
Source: Best Practices in Good Manufacturing Practice Excel: 100+ Good Manufacturing Practices (GMP) SOPs Excel (XLSX) Spreadsheet, SB Consulting